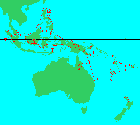
"Range of the genus Agathis Salisb. Figures above the hyphen indicate the number of endemic species, that below the hyphen the total number of species" (3).
Common Names
Kauri [Maori] (2).Taxonomic notes
Syn: Dammara (Rumph.) Lam. 1822.At this time (1999), taxonomic relationships within Agathis have largely been described in a series of publications ca. 1980 (4, 5, 6, 7, 8), 1988 (3) and 1998 (9, 10). These analyses have been based largely on morphology of the pollen cone and leaf cuticle, as foliar morphology is highly variable and female cones, which disintegrate upon drying, are seldom preserved (6). Work to date has shown a fairly high level of agreement in regard to the total number of taxa (21 species), a situation largely due to the high degree of endemism in the genus. The distribution of most taxa is confined to a relatively limited space within the vast archipelago reaching from Sumatra and the Philippines to New Zealand, that comprises the genus' range. Taxonomic investigations by several different authors (3, 6, 8, 9) have attempted to provide a infrageneric classification, but the several schemes presented to date show little agreement, place most species into one of three to five different groups, and fail to include more than two-thirds of the recognized species. With this in mind, I here present the most recent formal attempt at an infrageneric classification, that of de Laubenfels (3). De Laubenfels describes 3 Sections, largely on the basis of pollen and seed cone morphology. He assigns 12 species to these three groups; I have added the remaining 9 species based on his section descriptions.
Section Agathis (syn: Sect. Macrobracteatae Meijer Drees 1940) has pollen cones with spoon-shaped microsporophylls lacking angled creases, rarely sessile, with seed cones usually at least 7 cm long and seed bracts blunt along their apical margins. It includes 13 species of tropical and subtropical distribution (A. borneeensis, A. celebica, A. spathulata, A. lenticula, A. orbicula, A. philippinensis, A. flavescens, A. kinabaluensis, and I suspect (de Laubenfels does not enumerate them) A. atropurpurea, A. lanceolata, A. montana, A. corbassonii, and A. microstachya.
Section Rostrata de Laubenfels 1988 has leaves gluacous beneath, solitary resin canals between the vascular bundles; pollen cones sessile with microsporophylls spoon-shaped, more or less acute and spreading; seed cones spherical to oval, 5-6 × 6-7 cm; seed bract with a distinct projection or beak near the center of the ridged apical margin; seeds broadly oval with a blunt projection at one upper corner and a broad wing at the other corner. There are 3 spp., one each in New Zealand (A. australis), New Caledonia (I suspect A. ovata), and Borneo (A. endertii).
Section Prismatobracteatae Meijer Drees 1940 has microsporophylls with sharp creases dividing the apical part into 3 or more facies; pollen cones have have a short peduncle; seeds bracts are always blunt along their apical margins; leaves not glaucous beneath. It includes 5 species from New Guinea and New Britain to Queensland, New Caledonia and Vanuatu (New Hebrides) (A. labillardieri, A. robusta and, I suspect, A. vitiensis, A. silbae and A. moorei).
Description
Evergreen trees, usually monoecious, very large, with clear straight boles beneath a globular crown (young trees conical). Bark smooth and light grey to reddish, peeling with large thin irregular flakes that thicken on larger trees, leaving a pitted somewhat rough reddish brown surface, emits a milky sap when punctured. Branches horizontal or (when large) turning irregularly upward, leaving circular scars on the trunk after they fall. Buds usually globular, with few, imbricate, overlapping scales. Cotyledons 2, broad, lanceolate with an acute apex. Juvenile leaves larger than adult leaves, more or less acute, varying among the apecies from ovate to lanceolate. Adult leaves opposite, decussate, oval to linear, flat, broad, leathery, thick, with multiple parallel veins, sometimes glaucous beneath, with a short broad petiole, spirally arranged on main branchlets, opposite or alternate on side branchlets, reddish when young, turning dark green, leaving a rough cushion-like scar after they fall. Pollen cones appearing mostly on larger trees after seed cones have appeared, axillary (sometimes terminal), cylindrical, subtended at the base by rounded, sterile bud scales. Seed cones usually on short lateral branchlets, maturing and deciduous in 2 years, globose-ovoid, with numerous, flattened, broadly ovate scales without bracts and with minute apical umbos. Seeds more or less flattened and ovoid, one per scale, with 2 sometimes unevenly developed wings. Seed cone scales and seeds are asymmetrical with both dextral and sinistral forms produced (1, 3). The wood is straight-grained, pale straw to yellow-brown, with a specific gravity of about 0.47 to 0.61 (4).Range
Peninsular Malaysia to New Zealand, including Malesia, the Philippines, New Guinea, Melanesia and Australia. Their range extends from 10°30' N to 38° S, and from 96° E to 180° E. All species except A. australis are found within the Tropics. They occupy perhumid tropical rainforests as well as monsoonal semi-evergreen rainforest, which has a dry season of up to several months. They occur from near sea level to about 2500 m elevation, and in lowland situations they are found on diverse substrates including podzolized sands, ultramafics, carbonates and silicates. They often occur as discrete emergent trees, but sometimes form pure stands or are mixed in the lower canopy (4).Big Tree
In contrast to most other good-sized conifer genera, most species of Agathis form large trees; many species are the largest trees found in their respective forest ecosystems. The largest, and one of the largest of all conifers, is Agathis australis.Oldest
See Agathis australis.Dendrochronology
See Agathis australis.Ethnobotany
Thoughout its range, Agathis is highly sought after as a source of attractive, stright-grained, easily worked timber (4). Due to its relative scarcity and premium value, it has now been largely logged out and current production is almost wholly derived from plantations.The inner bark exudes a translucent or white resin, soluble in alcohol, called Manila Copal. This resin was formerly required for the production of many varnishes and of linoleum, and was harvested at the rate of 15-20,000 tonne per year from the 1920s to 1940s; however, the market has now been almost wholly replaced by synthetic substitutes (4).
Observations
Remarks
Many species of Agathis are listed by the World Conservation Monitoring Centre - Trees database as vulnerable or endangered and in decline. The WCMC list notes that "Agathis species are distinctive, highly sought and exploited for their valuable timber."Agathis is Greek for a ball of thread, an allusion to the globose female cone. Kauri is a Maori word, applied by that people to Agathis australis and generalized in modern usage to all species of Agathis (2).
Based on data from several wide-ranging species, Agathis participates in vesicular-arbuscular mycorrhizae; one associate is the phycomycete Endogone (4).
Known pests include the seed-eating moth Agathiphaga, found in Queensland and the New Hebrides. In Queensland it is attacked by the kauri coccid, coniferococcus agathidis, a defoliator which can weaken the tree, leaving it open to attack by boring beetles such as Euthyrrhinus meditabundus. The kauri thrip Oxythrips agathidis is another, somewhat less destructive defoliator also known from Queensland. Naturally, various species are also attacked by a wide range of fungal diseases (4).
Citations
(1) Silba 1986.(2) Boland et al. 1985.
(3) de Laubenfels 1988.
(4) Whitmore 1977.
(5) M.R. Bowen and T.C. Whitmore. 1980. A second look at Agathis. CFI Occasional Paper 13, Commonwealth Forestry Institute, Oxford University. 19p.
(6) T.C. Whitmore. 1980. A monograph of Agathis. Plant Systematics and Evolution 135: 41-69.
(7) T.C. Whitmore and C.N. Page. 1980. Evolutionary implications of the distribution and ecology of the tropical genus Agathis. New Phytologist 84: 407-416.
(8) C.N. Page. 1980. Leaf micromorphology in Agathis and its taxonomic implications. Plant Systematics and Evolution 135: 71-79.
(9) Anonymous. 1998. Wollemi Pine (Wollemia nobilis) recovery plan. Hurstville, NSW: New South Wales National Parks and Wildlife Service. 69p.
(10) Farjon 1998.
[Agathis] [Araucariaceae] [home]
This page is from the Gymnosperm Database
URL: http://www.geocities.com/~earlecj/ar/ag/index.htm
Edited by Christopher J. Earle
E-mail:earlecj@earthlink.com
Last modified on 20-Apr-1999