Soil Components
Solid supportOrganic - chemicals with carbonAir including oxygen
Inorganic - chemicals without carbon
Water
Organisms!! 1 acre-foot = 3 tons of organisms!
Inorganic particles
| Rock Gravel | ignore these for now but important in New England
| Size | Drainage | Water | Retention Air | Nutrient | Capacity Sand | 1-0.1 mm | +++ | 0 | +++ | 0
| Silt | 0.1-0.001 | + | ++ | ++ | ++
| Clay | < 0.001 | 0 | +++ | 0 | +++
| | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Need a mixture of these for optimum soil texture = LOAM
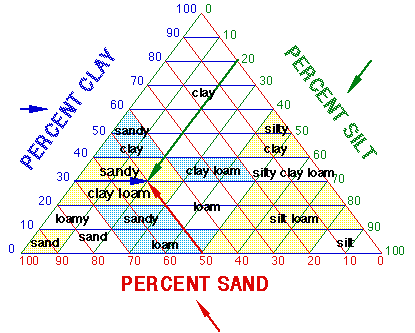
How to determine soil texture/type:
Calgon = Surfactant = surface active agent
= sodium hexametaphosphate
breaks up clumps......separates particles
sand first
then silt
then clay
Here is an example:
| Layer Height | Total Height | % of Total | Soil Type | What it means |
|---|---|---|---|---|
| Clay 3.0 cm Silt 2.0 cm Sand 5.0 cm | 10 cm | 30% Clay 20% Silt 50% Sand | Sandy Clay Loam | = drainage + air = nutrients = good for plants |
SOIL TRIANGLE

Organic Particles
rotting material = humussources
- improves drainage
- improves nutrient holding capacity
- improves water holding capacity
- leaves
- grass clippings
- compost
- green manure
- manure - esp. Horse
Nutrients Needed
| Macronutrients: | CHO air water | PK(i)N limited in soil | S Ca Fe Mg -------common in soil-------- |
|---|
Ca Mg found in lime
Micronutrients: Mo Cu Zn Co Mn Si B Al Cl etc.
trace amount needed usually found in soil
Fertilizer: Nitrogen - Phosphorus - Potassium (NPK analysis)
balance important:
20-20-20 a common general purpose fertilizerunbalanced fertilizers:10-20-10 common flower or fruit garden ("vegetables")
(phosphate needed for reproduction)
40-0-0 urea or ammonium nitrate formake your own mixture for exact needs BASED ON SOIL TEST!
lawn and true vegetables
(only nitrogen needed for reasonable growth)
(economical)0-40-0 super phosphate
0-0-30 potash
Avoid pollution caused by over-use of fertilizer (eutrophication)
Nutrients available only through WATER in the soil:
Water Dissociates:
H2O ------> OH- + H+ 10-7 M = distilled waterpH Scale 0 to 14:pH = - log [H+] = 7 for distilled water
100 10-7 10-14 M H+ ConcHOW TO ADJUST pH:
0-----------------------7-------------------------------14 pH
acid
sour
vinegar
cranberriesneutral base = alkalai
bitter
soap
beans & asparagus
How Acid affects nutrients available:
raises pH lime = Ca(OH)2 -----------> <------------- Al2(SO4)3 aluminum sulfate lowers pH
Clay Particle has negative charge
Opposites attract, so metal ions with positive charge stick:
Look like this in most soil!Acid rain = H+ This smaller ion replaces large metal ions
(cation exchange)

Released Cations available for uptake into roots or leach out!
Roots secrete acid, retrieve ions, retrieve acid ions.
Soil Horizons:
| black | A | leaching zone organics here - most roots | these layers lost by erosion, esp tropical soils |
|---|---|---|---|
| "red" | B | accumulation zone nutrients collect here | |
| rocky | C | weathered bedrock | |
| solid | D | bedrock |
Erosion Prevention:
Go Back to the Course Schedule.