Botany online 1996-2004. No further update, only historical document of botanical science!
The coleoptiles of grasses (like that of oat, the Avena - coleoptile) are popular test objects of plant physiology. A growing coleoptile that is illuminated unilaterally does grow towards the light source. This behaviour is common among plants and is known as phototropism. C. DARWIN (assisted by his son Francis DARWIN) attributed in his work "The Power of Movement in Plants" (1880) a decisive function in the recognition of a light stimulus to the coleoptile’s tip, and he observed that the actual bending occurs in a zone below the tip. He concluded that a transmission of impulse had to take place in the tissue. Studies of plant anatomical nature revealed that the growth towards light is caused by an elongation of the cells at the side that is shielded from the light. The phototropic reaction does not happen if the coleoptile’s tip is removed, though it can be induced again by the replacement of the tip. This indicates the existence of a substance that is spread from tip to bottom (basipetal direction) and that causes the elongation.
The Danish botanist P. BOYSEN-JENSEN interrupted the assumed substance flow by inserting a mica sheet into the shielded side thus separating the coleoptile’s tip from the tissue below (1913). The water-impermeable sheet interrupted the phototropic reaction. Consequently occurs no transport of the effector around the small tile. The phototropic reaction remained intact when the mica sheet was inserted into the illuminated side or along the coleoptile’s vertical axis.
In the late twenties was the material nature of the effector finally proved by the Dutch plant physiologist F. WENT. He assumed that a substance that flows from tip to bottom should also flow through a small cube of agar. In order to test his assumption did he place cut coleoptile tips with the cutting side on top of small cubes of agar. Some time later did he remove the tips and placed the agar cubes that he believed to contain the effector onto the decapitated coleoptiles. He wrote about the carrying out of the decisive experiment:
"When I removed the tip after an hour and placed the agar cube on one side of the seedling, nothing happened at first. But in the course of the night, the stump started to curve away from the agar block. It had acquired the capacity of the stem tip to grow! At 3:00 A.M. on April 17, 1926"
(according to F. B. SALISBURY, C. W. ROSS: Plant Physiology. Belmont/Cal: Wadsworth Publ. Comp. 1978, 2. edition.)
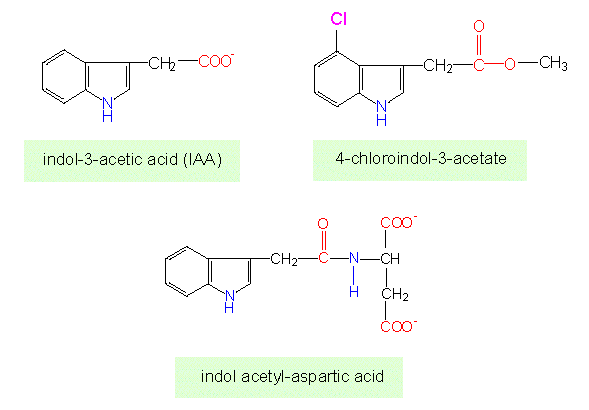
Went called the effector auxin (or growth-regulating substance). Its chemical name is indole-3-acetic acid (IES). The formula shows that it is a tryptophane derivative.
It turned out that auxin is a collective name for several similar compounds. Auxin occurs in cells in concentrations of 10-8 – 10-6 Mol/l. As we know today are IES and its similar compounds very common in green plants and fungi. Methyl-4-chlorindole-3-acetic acid and indole aspartate, for example, were found in unripe pea seeds (and unripe seeds of other plants, too). Auxins are often glycosylated or bound to proteins.
The rates of production and degradation as well as the ability of IES to be modified determine the concentration of the physiologically active compound within the cell. During the last years have a number of IES-analogous substance been synthesized and tested for their hormone abilities. It turned out that a hormonally active substance has to have three structural properties:
- The molecule has to contain a ring system with at least one double bond.
- The double bond has to be adjacent to a side chain.
- A carboxyl group that is separated by one or two C-atoms is required.
These conditions led to several clues about the structure of the binding site(s). The receptor has accordingly to have two separate contact sites.
The distribution of auxin within plant tissues is subject to clear and recognizable rules that indicate a transport simultaneously active and polar. This means that IES-specific carriers have to exist beside the receptor (or receptors). At least six reasons speak for this:
- Transport occurs always directed: it is polarized.
- The transport velocity is higher than expected from simple diffusion.
- Transport can occur against a concentration gradient.
- Transport is energy-consuming and is drastically reduced in the absence of oxygen.
- The transport system is substrate-specific. It transports certain auxin molecules like IES or naphtylacetic acid faster than 2,4-dichlorphenoxy acetic acid, for example.
- The transport system can be blocked by specific inhibitors.
What is the biological significance of auxins? In lower concentrations do they aid the coleoptile’s elongation, that of the shoot and the roots. If the concentration becomes higher, the effect reverses and elongation of root and shoot is inhibited. The reason is a stimulation of ethylen production, a gaseous hydro-carbon that is a plant hormone, too. One of its effect is the inhibition of elongation. Auxins are involved in the differentiation of vascular bundles, they control abscission, induce beta-1,4-gluconases in pea roots, and stimulate the opening of tree buds as well as the rapid growth of young shoots. They do also increase the rate of cell division within the cambium, i.e. they stimulate secondary thickening. Furthermore do they aid the development of ovary into fruit, and they are responsible for the evolvement and the maintenance of apical dominance.
Auxins increase the plasma current, the plasticity of the cell wall, and they cause a proton efflux out of the cell. This list of activities is far from being complete, but it shows how varied the effects of auxins are.
At last a question about the possible mode of action: it has been mentioned at the beginning that auxins effect several different primary processes of the cell. Experiments show that they: sie
- increase the rate of transcription.
- Control the activity of certain enzymes, and
- have an influence on the ion pumps within the membrane.
Model studies with isolated membrane vesicles (like isolated vacuoles) have been carried through in order to understand the influence of auxins on membranes. Experiments with plasma membrane vesicles showed that the accumulation of auxin is dependent on pH and electron potential. The transport of auxins through the membrane is directed. Auxin specifically binds tonoplasts, and it influences the release of calcium ions from the vacuole in vitro. Different carriers for import and export have been detected: a proton carrier (S) that causes symport and an auxin-anion-carrier (AC) that is an active antiport-carrier. Moreover was it demonstrated that these auxin carriers are distributed asymmetrically within the membrane.
© Peter v. Sengbusch - Impressum