Botany online 1996-2004. No further update, only historical document of botanical science!
|
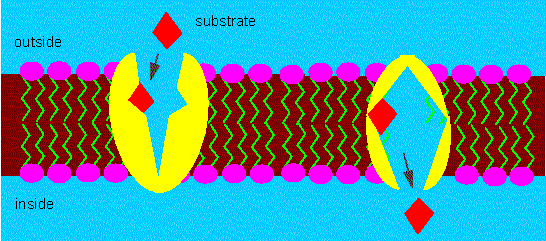
Participation of a carrier molecule in the active transport of certain molecules through the membrane. The transport process is specific and energy-consuming. The conformation of the carrier changes during transport (ping-pong mechanism).
Active transport is the movement of a molecule across a membrane or another barrier that is driven by energy other than stored in the concentration gradient or the electrochemical gradient of the transported molecule. This type of transport requires usually the expenditure of ATP and the help of specific transport proteins. In this way can even large molecules can be channelled through the membrane. For the understanding of the details of the mechanism are both the structure of the involved transport molecules and the question how the energy for the transport is supplied and how it is used important. We will meet the electrical membrane gradient again in this context.
Active transport can only occur at intact, closed membranes. Such membranes can envelop very different compartments, like the whole cell, vesicles, the vacuole, the mitochondrial matrix, the inner thylacoid space of the chloroplasts, etc. As a result of active transport can ions and metabolites be concentrated within the respective compartment or the cell and the steady state of the metabolism can be kept constant despite of large fluctuations in the external medium's composition. Ions, especially potassium, calcium, magnesium and phosphate have an important part in the regulation of the metabolism.
The transport direction is thermodynamically determined by coupling the transport with a gradient, usually an electron gradient. The direction can be reversed if the appropriate substrate concentrations are chosen.
As a consequence of the respiratory chain and of photosynthesis are protons channelled out of the mitochondrial matrix and the thylacoids of the chloroplasts, respectively. At the same time causes the efflux of protons a translocation of electric charges. That way is both a chemical proton gradient and an electric potential generated. The tendency of the protons (H+) to return to the inside of the compartment is therefore quite large. It is called the proton motive force. P. MITCHELL (1966, 1974) studied the process and his observations pointed at a key position of the proton translocation. The energy set free when the protons flow back is used for the synthesis of ATP, the involved enzyme is the ATP synthethase. Depending on whether the reaction takes place in mitochondria or in chloroplasts is it spoken of oxidative phosphorylation and photophosphorylation, respectively. But protons can flow back under two further conditions:
By antiport, i.e. a proton is exchanged with another cation and fuels in that way the transport of its counterion out of the compartment.
By symport. A proton is transported into a compartment and the energy for the transport is supplied by the simultaneous transport of an anion or a substrate molecule in the same direction. The process allows the uptake and accumulation of anions or (small) molecules by the compartment. In other words: an electrochemical gradient is used as a source of energy for the active transport of molecules and ions through the membrane. A second possible energy source for transport processes is the breakdown of ATP.
ATP hydrolysis is actually the reversion of oxidative or photophosphorylation. The ATP synthethase works the other way round: protons (and other cations like potassium) are actively transported out of the compartment (proton pump) and build as a consequence a pH gradient and a membrane potential up. These two forces again drive the translocation of other molecules (or ions) via antiport or symport. As mentioned at the beginning is active transport the name of two principally different but coupled processes:
The transport performed by a carrier. The direction is solely dependent on the substrate gradient and serves to even out substrate concentrations.
The energy-dependent process that leads to an asymmetry of the transport so that the transported molecules are accumulated at one side of the membrane against an (electro-) chemical gradient.
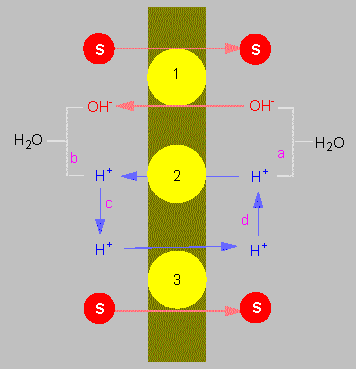
Chemiosmotic coupling of the transport of molecules through a membrane according to the principles of antiport (1) and symport (2, 3). S = substrate
(according to C. L. SLAYMAN und D. GRADMANN, 1975).
The resulting asymmetric transport of the carrier is based on an energy-consuming conformational change of the molecule (for example a phosphorylation of the carrier). The two states of conformation (phosphorylated and non-phosphorylated) are characterized by different affinities for their substrates. The velocity constants of the permeation can vary by scales. A number of toxic substances like ammonium ions or dinitrophenyl (DNP) destroy the proton gradient so that no more energy is available for active transport. Such substances are called uncoupling agents.
The membrane-bound proteins needed for active transport are without exception oligomerous complexes, i.e. they consist of several protein subunits. They are often termed pumps, too.
A reversible ATP synthethase (able to hydrolyze ATP) that gets its energy from the electrochemical potential difference between inside and outside is among the most important components of the whole complex. The transport kinetics of the pumps can - similar to those of facilitated diffusion - be compared with enzyme kinetics.
The proton complexes work analogous to enzymes and are therefore often also classified as permeases. Proteins of animal membranes (like erythrocytes) and micro-organisms have been studied especially extensively.
The number of polypeptide chains that make up a complex, the molecular weight and the number of ions or molecules transported per time unit is often known. It is also known, that many pumps work only when in their specific surrounding, in other words when in close vicinity to the right phospholipids. And finally is it known that pumps are integrated into the membrane in always the same orientation because transport is always directed.
But in no case is the exact tertiary or the quaternary structure of the complex known and only these structures would explain, why a specific molecule or ion and no other, similar ones are transported.
The best-known and best-studied ion pumps are the sodium-potassium- and the calcium pump. The sodium / potassium pump was isolated from the most different types of membranes.
It is generally known that cells contain more potassium than sodium ions. This is also true for plants living in saline (sodium containing) soils. Such plants are also said to be halophilic. Especially in the cells of halophilic plants was a very large activity of sodium / potassium exchanges detected. The cells invest a considerable amount of energy in order to maintain within the cytosol as low a concentration as possible. Part of the sodium is pumped into the vacuole where it does not interfere but helps to maintain the osmotic pressure. A number of secondary plant products like the glycosides oubain (strophanthin) and digitonin inhibit the sodium / potassium pump selectively.
The calcium pump located especially in intracellular and organelle-membranes is of no lesser importance. The calcium concentrations within the different compartments differ by scales. On the other hand is calcium known for its regulating effects on the metabolism. The calcium pump has accordingly a direct influence on the throughput ratios of many biosynthetic pathways.
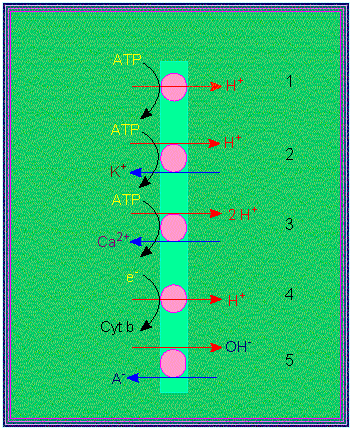
Proton pumps: 1. electrogenic pump, 2. electro-neutral pump, 3. electro-neutral pump with calcium as its counterion, 4. electrogenic proton transport, 5. electro-neutral anion / OH antiport (according to R. E. CLELAND, 1982).
Our knowledge about the exact distribution of ions within the single compartments is rather sketchy, especially in plants.
A large advantage of micro-organisms is that mutants with defects in the transport system can be isolated. The defects can be traced back to the losses or the changes of particular subunits of the protein complex. Two principally different sugar transport mechanisms were detected in micro-organisms:
Active sugar transport via indirect coupling to an energy source. The substrate is transported through the membrane without changes. The carrier changes its affinity for the substrate during its translocation through the membrane. The affinity is high at the outside of the membrane and low at its inside. In many cell types is the transport of metabolites (and substrates, respectively) coupled to a functioning sodium / potassium pump.
Active sugar transport by means of substrate modification. The substrate is chemically modified during the transport. Examples are the phosphorylation of sugars and the glycosylation of adenine. The process is also called vectorial phosphorylation. The transport is initiated by a chemical reaction during which the substrate is 'phosphorylated into the cell'.
© Peter v. Sengbusch - Impressum