Botany online 1996-2004. No further update, only historical document of botanical science!

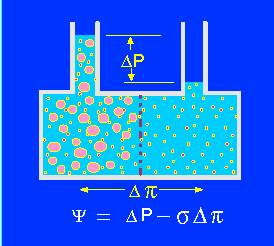
Model of an osmotic system. Two compartments are separated by a semi-permeable membrane. The size of the pores is large enough to let small particles (ions, molecules) pass freely though small enough to inhibit the passage of larger molecules. In the left compartment develops a higher osmotic pressure due to the hydration sheath that surrounds each particle. Since it contains more 'water-binding' particles, expands the volume of the left compartment. The osmotic pressure (turgor) can be measured with a pressure gauge. It depends on psi = water potential, delta P = hydrostatic pressure, sigma = the ratio of the apparent osmotic pressure to the theoretical osmotic pressure for the semipermeable membrane and pi = osmotic pressure of the compartment (cell).
Biological membranes are usually permeable for water and gases like oxygen, nitrogen, carbon dioxide and others and semi-permeable for some ions, sugar and other small molecules. They are, with some exceptions, impermeable for large molecules. When disregarding mediated transport (transport occurring through the action of specific carrier molecules) is the permeability of the membrane dependent on its pore size.
Osmosis is the net flow of water through a semi-permeable membrane that is driven by a difference in concentration of solute on either side. A model will illustrate this: At a time t0 contain two equal volumes (v1 and v2) a dissolved substance. It has the concentration c1 in volume v1 and c2 in volume c2. Let us assume that c1 is twice c2 and that both volumes are separated by a flexible membrane that is permeable by water but not by the dissolved substance.
At a time t1 would under these circumstances a movement of the membrane be observed. The concentration c1 would equalize with c2 since water is able to permeate freely through the membrane. The volume v1 would at equilibrium be twice v2. The more concentrated solution would have gained volume.
That is, osmosis causes a net flow of water from a solution with a high to one with a lower water-potential. The water potential (Psi) describes the flow of water from solutions of low concentrations to solutions of higher concentrations. Water flows only in the direction of a lower Psi or, in other words, it flows along a decreasing Psi-gradient. The process is exergonic, i.e. it needs no energy but releases it. The reverse process during which water is transported against a decreasing Psi-gradient is consequently endergonic.
Osmosis procures a hydrostatic pressure on a membrane (also called osmotic pressure or osmotic potential, p, with the dimension atm or Bar). It is dependent on the concentration of solutes as the model has shown. It is therefore also spoken of a solute potential. The osmotic pressure helps to reduce the water potential. It is solely dependent on the number (and not the type) of dissolved particles, that is, of the molarity.
A solution that is separated from another solution by a semi-permeable membrane can have three osmotic states:
In an isotonic solution is the pressure at both sides of the membrane the same.
A hypotonic solution has a lesser number of solute particles than the solution to which it is compared, while
a hypertonic solution has a higher number of solute particles. At equilibrium is a solution always isotonic.
Each cell has constantly to cope with osmotic phenomenons. Cells without walls that live in aqueous solutions are usually hypertonic. They are subject to a continuous influx of water that results in a pressure on the inside of the membrane. Some ciliates (like Paramecium) and flagellates (Euglena) have a special organ, a pulsating vacuole, that pumps the water out again and has to be supplied with energy. Red blood cells (erythrocytes) occur normally in isotonic surroundings (blood plasma). The cells burst if the blood is diluted with water since their membranes cannot face the osmotic pressure within the cells. Blood is therefore always diluted with an isotonic (0.9%) NaCl-solution.
The situation in plant cells is different in that they are enclosed by a rigid cell wall. In a hypotonic solution can they only take up water until the inner and the outer water potential are equal. The solution at the inside of the plant cell is subject to an additional hydrostatic pressure. Plant cells store ions, sugars, organic and amino acids and other substances in considerable concentrations in their vacuoles. The solutes cause an influx of water. In this way can plant cells build up a large positive internal pressure, the turgor pressure. It has a decisive influence on the maintenance of the rigidity and stability of plant tissues. Each cell exerts a pressure on its neighbouring cells. The pressures add up to a large tissue tension.
Plants that loose water wilt because their turgor pressure decreases. The stability of the tissues cannot be maintained. As long as the cells are still living can the turgor pressure be fixed again, a phenomenon common in everyday life: wilted plants recover after watering.
Osmosis and turgor pressure have been extensively examined at the end of the last century by W. PFEFFER. He constructed a model (an osmometer, the PFEFFER cell) that allows the quantitative determination of the osmotic pressure. He expressed the relation between turgor and osmosis in a formula that describes the osmotic state of the cell:
Sz = Oz - W
where Sz is the suction force of the cell, Oz its osmotic value and W the wall pressure.
If plant cells are given into in a hypertonic solution (a 5% potassium nitrate solution, for example), water is extracted from the protoplasm, it shrinks. This phenomenon is called plasmolysis. The process is reversed as soon as the cells are transferred into a hypotonic solution (deplasmolysis).
Since the end of the sixties can the cell walls and the tissues of the mesophyll of a number of plant species be dissolved with a treatment with pectinase and cellulase. The result are cell wall-free protoplasts that can only be cultured in isotonic media (for example a 0.6 - 0.7 molar mannitol solution).
© Peter v. Sengbusch - Impressum