Botany online 1996-2004. No further update, only historical document of botanical science!
Alkaloids are a group of nitrogen-containing bases. Most of them are drugs. Only a few (like caffeine) are derived from purines or pyrimidines, while the large majority is produced from amino acids.
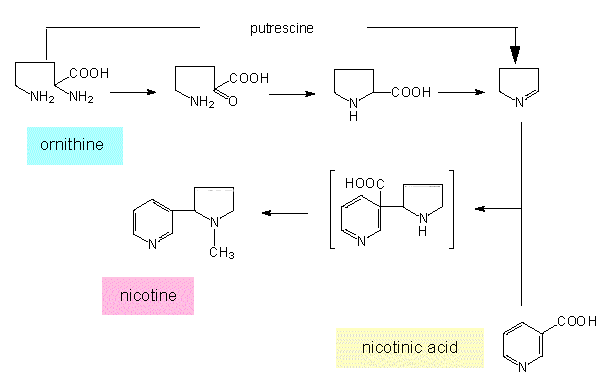
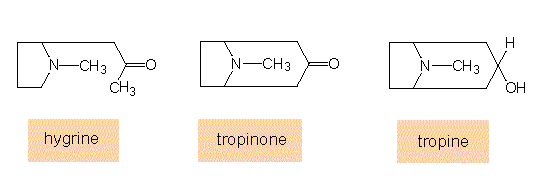
Ornithine derivatives: Ornithine is a precursor of the cyclic pyrrolidines that occur in the alkaloids of tobacco (nicotine, nornicotine) and other Solanaceae. Nicotine is a starting compound of numerous further tobacco alkaloids. During the biosynthesis of tropane, intermediates are produced that are at the same time starting compounds for cocaine and hyoscamine.
|
|
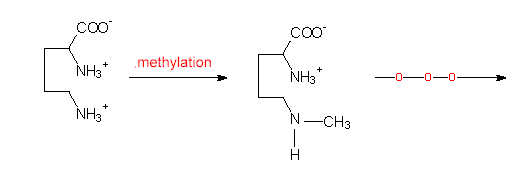
Biosynthesis of tropane: The starting compound of this synthesis is ornithine, methylornithine is the first intermediate.
A further group is constituted by the pyrrolizidine alkaloids: Biosynthesis of The Basic Structure of Pyrrolizidine Alkaloids
Lysine derivatives: Lysine is the precursor of piperidine that forms the skeleton of several alkaloids. Among them are the bitter principles of the lupine, lupinine and lupanine.
Lycopodine, a substance obtained from clubmoss (Lycopodium) belongs to this group, too. This is mentioned only to show how little the plants are related that are the producers of very similar substances.
Derivatives of phenylalanine: The most important are:
Ephedra-alkaloids: ephedrine, pseudoephedrine,
alkaloids gained from micro-organisms: cytochalasine B and D,
the Taxus-alkaloids: taxine,
the Lunaria-alkaloids: lunarine and lunaridine,
alkaloids of the Lythraceae.
Tyrosinederivates: Synthesis of Benzylisoquinolines, Starting with Two Mol Tyrosine
Moreover, a number of aromatic compounds containing carboxyl-groups is incorporated into other alkaloids like, for example, atropine (an alkaloid of Atropa belladona).
During the last years Cytochalasine has gained importance in cell biology. It inhibits movements performed by microfilaments and is thus a suitable inhibitor for processes that involve actin.
Tyrosine derivatives: Tyrosine is the starting product of a large family of alkaloids. The first important intermediate is dopamine which is the starting product of the biosyntheses of berberine, papaverine and morphine, too.
Morphine synthesis: Two tyrosine rings condense and form the basic structure of morphine that is subsequently modified.
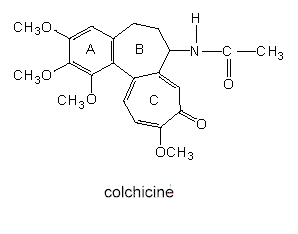
The formulas above represent prototypes of which a range of similar compounds (like, for example, the opium alkaloids, codeine, etc.) are derived. Colchicine, an alkaloid of the naked ladies (Colchicum autumnale and the related species C. byzanthinum) belongs also to this class. It is a poison best-known as an inhibitor of mitosis. It prevents the aggregation of tubuline dimers so that no micotubuli are formed.

Indole alkaloids (derivatives of tryptophane, etc.): The analysis of this group has proceeded very well during the last years. Besides chromatographic techniques, especially mass spectroscopy and nuclear magnetic resonance (NMR) have been used. About 1200 different compounds all of which are tryptophane derivatives have been isolated until today. They correspond to 25 percent of all known alkaloids. Only few of them are known by a broader public although many of them are used by medicine.
Among the tryptophane-derivatives is d-tubocurarine, one of the active components of curare, the arrow poison used by South American Indians. The Apocynaceae, Loganiaceae and Rubiaceae families are characterized by a broad range of indole alkaloids of which the Vinca-, Rauvolfia- and Cataranthus - alkaloids are examples. They are generated by coupling iridoids, very common compounds derived from monoterpenes to indole that is based on tryptophane. A lot of well-known fungi poisons belong to this group.
Isoprene derivatives are examined closer in the next section. Only a few derivatives that are counted among the alkaloids due to the nitrogen they contain shall be mentioned here. In contrast to the amino acid derivatives that contain nitrogen right from the beginning, it is incorporated much later here. Gentianine as well as further toxic compounds like, for example aconitine obtained from Aconitum and Delphinum belong to this group. An important sub-group is formed by the Solanum- alkaloids that can be found in nearly all organs of Solanum- (potato) and Lycopersicon (tomato) - species but occur also in very different taxa (lilies and Asclepiadaceae).