
Botany online 1996-2004. No further update, only historical document of botanical science!
The most important problem in this context is:
how are fatty acids produced?
Acetyl-CoA is most important in this
context. We did already get to know it as an intermediate connecting
glycolysis and the citric
acid cycle. There seem to exist further biosynthesis pathways in
chloroplasts, but they are still pretty unclear to us. It can also be
produced from citrate under consumption of ATP in the cytoplasm (the
reversion of the first step of the citric acid cycle).


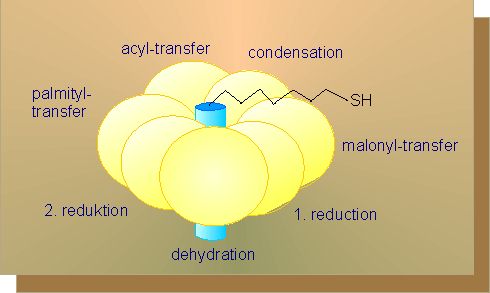
To start fatty acid synthesis, acetyl-CoA is transduced into malonyl-CoA by addition of carbon dioxide. The co-factor necessary for this step, a protein complex, is localized in the thylacoid membranes of the chloroplasts of green plants and seeds. To proceed with fatty acid synthesis, both acetyl-CoA and malonyl-CoA are needed. Both residues are transferred to an acyl-carrier protein (ACP), each under splitting off of CoA.
They condense in this activated state while carbon dioxide and one of the ACP residues is cleaved off. The step is preceded by a reduction, a dehydration and again a reduction. In both reductions, the electrons are provided by NADH + H+. The resulting product is butyryl-ACP.
The butyryl residue differs from the acyl residue by two additional -CH2-groups. The butyryl-ACP is coupled to malonyl-ACP and the whole chain of reactions is run through for a second time. As a result, a fatty acid residue is gained that contains two more groups of -CH2 than the butyryl residue. Again malonyl-ACP can be bound and the reaction chain be followed so that another C2-unit is added to the fatty acid residue. This may be done several times and each time, two -CH2 groups are added. This explains why the number of carbon atoms in fatty acids is always even. The synthesis stops as soon as sixteen carbon atoms are present (C16). The preliminary end product of fatty acid synthesis is accordingly palmitic acid. Further elongation of the molecules are achieved by other mechanisms.
The reactions outlined here take all place at a multi-enzyme complex, the fatty acid synthethase (see above). It is an enzyme with several active centres. It has only two polypeptide chains in yeast that are encoded by a single gene. The separation into two chains occurs during their synthesis. The ACP is anchored to the centre of the complex and functions as a wheel.
I.e. the substrate at its outer end, at first a malonyl residue, is successively turned along the single active centres so that the chain elongated by a C2-unit finds itself in the start position again after one circuit. The big advantage of such a multi-enzyme complex is in avoiding of losses caused by diffusion and the speedy passing on of each intermediate to the next neighbouring active centre. The procedure does also save energy.
Many fatty acids contain double bonds (= unsaturated fatty acids). They are produced from the saturated fatty acids and the surplus hydrogen is transferred onto NAD+. The formation of the double bond occurs strictly stereospecifically. Most reactions result in a cis-linkage, but there exist also enzymes that can generate trans-bonds.
Beside the just outlined classic way of fatty acid synthesis, a number of other pathways have been found in different organisms that use other intermediates or short unsaturated fatty acids. Dodecatrienic acid, for example, (12 C atoms, three double bonds) can be elongated by three acetyl units and thus be transferred into linolenic acid (18:3). This pathway was proven in mitochondria and the cytosol but it is very likely that it occurs also in chloroplasts. Different amounts of 18:3 and 16:3 fatty acids were found within the glycolipids of different plant species. It is therefore distinguished between 18:3 plants whose fatty acids with 3 double bonds are always 18 C atoms long and the 16:3 plants that contain both C16- and C18-fatty acids. The ratio of 16:3/18:3 is subject of species-dependent variation.
Share of C16- and C18-fatty acids in monogalactosyl diglycerides in 18:3 plants (above) and 16:3 plants (below). Each lipid contains two fatty acid residues. At least one of it is a C16-fatty acid (according to J.P. WILLIAMS et al., 1983)
The flux of lipids between the membranes of different compartments. In green plants, fatty acids (FA) are always synthesized within plastids. Phospholipids (PL) are mostly produced in the endoplasmatic reticulum (ER) and to some extend also in the mitochondria (according to T.S. MOORE and G. D. TROYER, 1983)
Fats are produced from fatty acids by transfer of the fatty acid residue onto glycerine. During the reaction, ACP is cleaved off and a new ester bond is generated (the linkage to ACP was also an ester bond). The single types are grouped into three different classes according to differences in their syntheses:
Group A is represented by the glycolipids. The are the largest
group of chloroplast lipids. Lipids of group B are linked to
nucleotide diphosphate coupled alcohol. They represent the largest
group of lipids in mitochondria and cytoplasmatic membranes. Their
synthesis occurs in the cytosol. Group C consists of nucleotide
diphosphate diglycerides that are linked to an alcohol. The main
place of synthesis of this group is a microsome fraction (in the
cytosol). Some reactions are likely to occur in mitochondria,
too.
This short outline points at the importance of the compartments as separate reaction spaces.
Catabolic pathways: the catabolic pathways of lipids are at least as important as the anabolic ones since many lipids are storage compounds. They serve as energy reservoirs and are activated upon need. The catabolism is not a reversion of the anabolism although C2 units are usually transferred to CoA during this process. It is spoken of a beta-oxidation. The involved enzymes belong to the lipase group. Protons and electrons are transferred to FAD and NAD+. Citrate acts as an activator of synthesis and as an inhibitor of the breakdown. It is only present in surplus amounts if too much acetyl-CoA is present. A citric acid cycle running too fast means that too many energy-rich compounds are used and transformed into energy that may not be needed any more. It is thus more economic to stop the breakdown of storage compounds and to start fatty acid synthesis by feeding acetyl CoA into the respective pathways.
In seeds and leaves, an alpha-oxidation exists besides the beta-oxidation. This pathways generates at first alpha-hydroxy acids. In a further step, carbon dioxide is cleaved off. The product is a fatty acid that is reduced by one C1 unit.
|
|
the synthesis takes place in the cytosol, breakdown in the mitochondrial matrix. |
|
|
The intermediates of synthesis are covalently bound to ACP, while the breakdown intermediates are coupled to a CoA. |
|
|
The enzymatic activities of synthesis are combined in a multi-enzyme complex, the fatty acid synthethase. The enzymes needed for the breakdown are structurally not linked. |
|
|
The fatty acid grows by a C2 unit in each round of synthesis. The elongation frees carbon dioxide. |
|
|
NADH + H+ is the proton- and electron donator of fatty acid synthesis |
|
|
Fatty acid synthesis ends with the generation of palmitic acid (C16). A further elongation of the chain and the generation of double bonds is caused by other enzymes. |
Other Lipids
The synthesis of an extensive group of plant lipids as well as compounds of a lipid nature start with isoprene. Numerous of the compounds generated this way cannot be counted among the primary metabolism. Further information about this topic can be found at: