Botany online 1996-2004. No further update, only historical document of botanical science!
'The loss of substance that is generated incidentally by the respiration has the aim to develop mechanical forces by which the atoms or molecules of the other substances are set in those motions that result in growth and other functions of the living plant. In other words: respiration is the source that fuels all phenomenons of life while the assimilation occurring in chlorophyll-containing organs generates the substances that are subsequently set in motion to serve life.' (J. v. SACHS, 1882)
All reactions of glycolysis take place in the cell's cytosol. The citric acid cycle and the respiratory chain are located in another compartment: within mitochondria at the inner mitochondrial membrane. It is here that ATP is generated under consumption of oxygen and this is why mitochondria are often said to be the cell' 'power plant'. The process is also called oxidative phosphorylation. It resembles photophosphorylation that is part of photosynthesis. Both processes need intact membranes that form intact compartments separated from the rest of the cell to function.
In summary, the respiratory chain can be characterized as the process during which NADH + H+ and FADH2 are oxidized under consumption of oxygen and water and ATP are generated.
The generation of water (oxyhydrogen reaction) is strongly
exergonic:
2 H2 + O2 > 2 H2O.
In the respiratory chain, this energy is gradually set free in a number of intermediate steps (an electron transport chain) and can thus be used for the generation of energy-rich compounds.
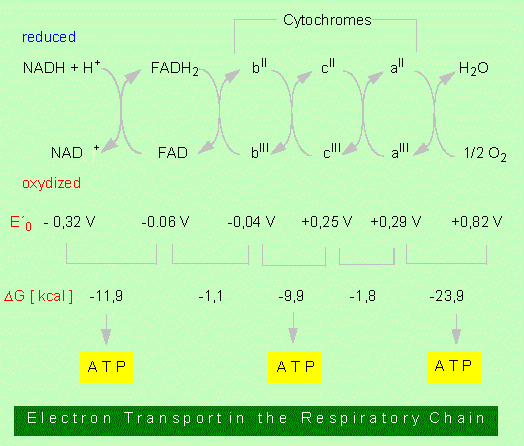
1. NADH + H+ is oxidized resulting in NAD+. The hydrogen is passed on to a further hydrogen-acceptor, the FAD.2. FADH2 is oxidized by cytochromes. Cytochromes (cytochrome b, cytochrome c, cytochrome a , etc.) are proteins with haeme-groups (porphyrin rings) as cofactors. Located in the centre of this ring is an iron ion of changing valency:
FeIII <> FeII
While FAD accepts both electrons and protons, cytochromes transport only electrons. The protons are given off into solution. In the respiratory chain, the FeIII of cytochrome b is at first changed into FeII. In plant cells, at least three different cytochrome b-types are in series. The iron ion of cytochrome b achieves its original FeIII state again by transferring one electron to cytochrome c which transfers it in a further step onto cytochrome a. A quinone/hydroquinone system may be inserted between the flavoprotein and the cytochromes.
3. The reduced cytochrome a transfers the electrons onto oxygen that reacts immediately with free protons to form water.
It will be shown in the discussion of redox-reactions that each redox pair is characterized by a redox potential (E'°). This potential is proportional to the free energy (delta G°) of the reaction. The values of the respiratory chain are given in the picture above. It can be seen that three redox pairs have enough difference in potential to drive the generation of one molecule of ATP each. Accordingly, three molecules of ATP , the equivalent of NADH + H+ while a molecule of FADH2 gives only two ATPs.
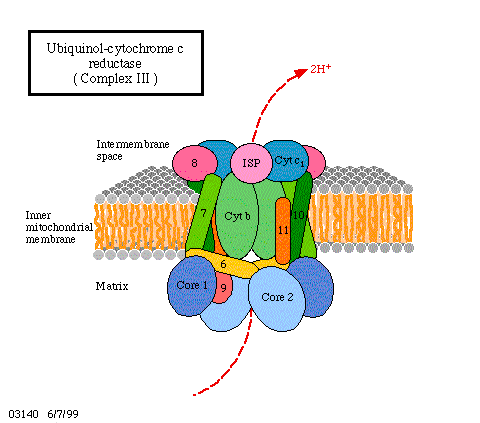
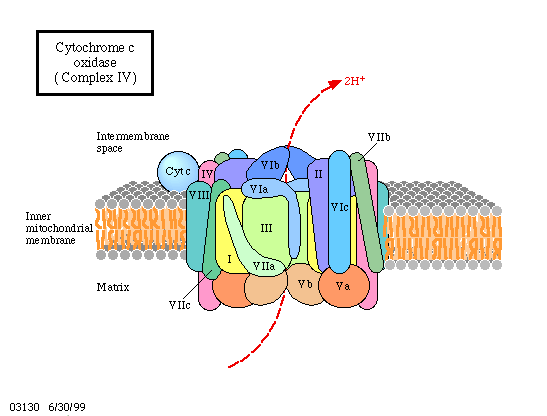
How does all this look on a mechanistic, molecular level? All the cofactors are bound to proteins and the proteins again are either integral components of the membrane or are closely associated. It is also important to know that all these proteins are oriented in the membrane in always the same way and that the membrane is impermeable for protons. Besides the mentioned proteins, one more enzyme is necessary, the ATP synthase. How is the activity of the ATP synthase coupled to the electron transport chain? Proofs exists since quite some time already that this enzyme functions itself as a coupling factor.
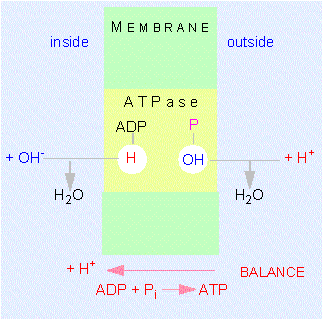
A model of the way protons are translocated through the inner mitochondrial membrane. Please notice that the protons are not passed on but that OH-ions are set free at the outside of the membrane while protons are given away into the matrix at the inner surface. This results in a directed movement of the protons. The ATP synthase (also known as ATPase) has two neighbouring binding sites for ADP and phosphate.
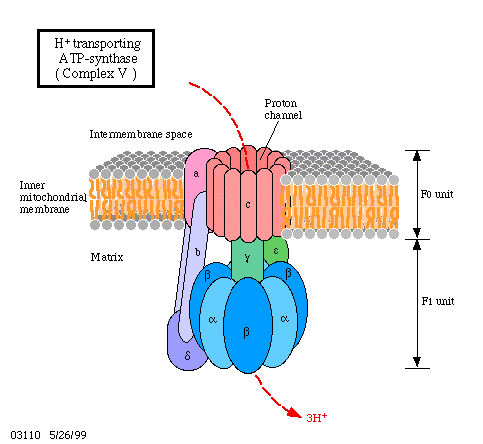
In 1961, P. MITCHELL postulated that protons are translocated through the membrane in a directed way. This results in a pH gradient.
The passage back occurs via a specific proton channel. This passage is coupled to ATP-synthesis, using the potential energy of the proton gradient for the formation of the third phosphate bond of ATP.
We can now calculate the end result of glucose degradation: the
oxidation is coupled to a decrease of the free energy; 686 kcal/mol
(= 2881 kJ/mol) are obtained by the complete oxidation of glucose.
How much of this energy can the cell use?
Six mol ATP per mol glucose are generated (substrate chain phosphorylation). This is because all steps after the breaking down of fructose-1,6-phosphate have to be counted twice (once for each of the two resulting C3 molecules), so it is 3 x 2 ATPs. Of these six ATPs, two are needed to start glycolysis. That leaves four.
During the course of glycolysis up to acetyl-CoA, 2 x 2 NADH + H+ are generated. An additional 3 x 2 NADH + H+ and 1 x 2 FADH2 are produced in the citric acid cycle. One NADH + H+ gives three, one FADH2 two ATPs when fed into the respiratory chain. This sums up to 34 ATPs plus the 4 ATPs of glycolysis. A total of 38 mol ATP are thus gained by the cell's degradation of one mol glucose. Since each energy-rich bond of ATP contains 7.3 kcal/mol (= -30.6 kJ/mol), the 38 ATP equal 277 kcal/mol (ca 1163 kJ/mol). This is 40.6% of the theoretically possible gain. The other 59.4 percent are set free as heat. This is a very high percentage compared to the gain of technical machines like steam or petrol engines that is around or below 20 percent.
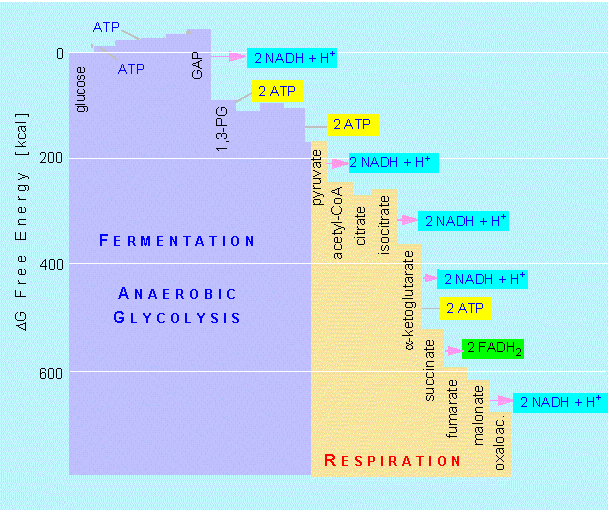
Decrease of free energy (delta G) during glycolysis and respiration.
Conversion factors: 200 kcal = 1680 kJ ; 600 kcal = 2520 kJ.