Botany online 1996-2004. No further update, only historical document of botanical science!
Aromatic hydrocarbons have ring structures. Ring systems with double bonds that cannot be localized are called mesomere systems. Aromatic hydrocarbons carrying hydroxyl-groups are called phenols. There exist also ring compound with ring elements other than carbon. Such ring systems are termed heterocyclic rings. Heterocyclic rings are components of nucleotides that have an important role in the cell.

Benzene and its derivatives belong into this group, some of whose members occurring in plants are characterized by a strange, 'aromatic' smell. The benzene molecule has a ring-shaped structure with six C-H-groups that are linked alternately with C-C single and C=C double bonds. The structure should thus be written (according to KEKULE):
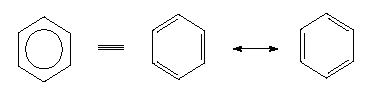
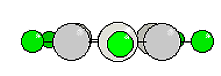
The electron theory distinguishes between two types of bonds in such a ring system, the sigma-bonds and the pi-bonds that are arranged vertically to each other and are conjugated so that the double bonds of such a system cannot be localized directly. Such a system is also called a mesomere system. There exist consequently six equal 'aromatic' bonds. A compound consisting of two benzene rings is naphthalene
It is found in anthracene and phenanthrene
Aromatic hydrocarbons with hydroxyl-groups are called
phenols. They are able to
dissociate in alkaline solutions and to form phenolates.

This group comprises molecules that contain other elements besides carbon as ring members. These elements are also called hetero-atoms. These compounds do, too, belong to a class of the aromatic hydrocarbons since their ring systems are also characterized by conjugated double bonds. Rings with five or six elements are most stable and thus most common. Interesting are on one hand the simple N-containing rings
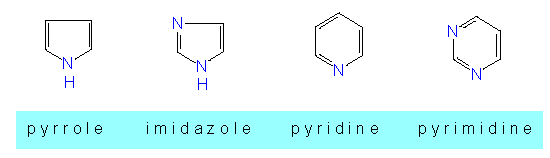
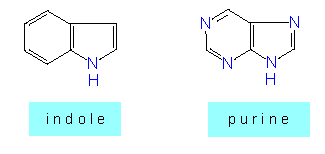
and on the other hand the condensed ring systems where several rings, for example a ring with five and another with six elements are condensed, as they are in
Among the more complex condensed rings is the nitrogen- and sulphur-containing thazole ring: It is an essential part of thiamine. thiazol
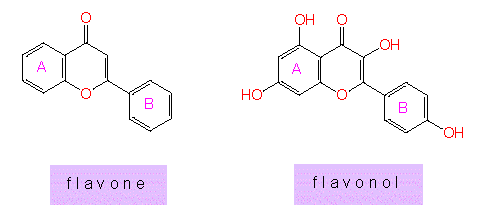
and flavon and flavonol that covers an oxygen-containing ring. The
ring systems in flavone and flavonol are usually termed "A" and
"B".
has also an oxygen in one of its rings (both are basic compounds of purple flower colours) as well as a combination of three condensed rings (a benzene-, a pyrazin- and a pyrimidine-ring), the
Isoalloxazine is a component of riboflavine. Heterocyclic rings form together or with other molecular groups components that cover essential functions in the cell.
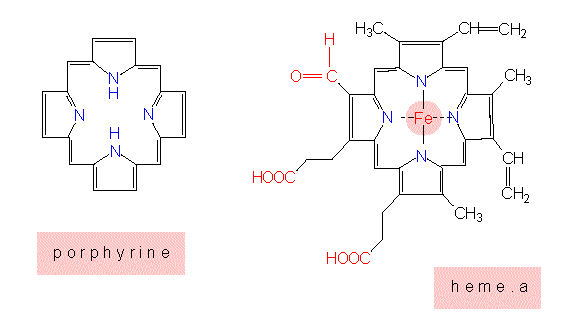
Pyrrol rings are the basic unit of porphyrine rings that again are found in chlorophyll and the heme groups of the cytochromes
Purine and pyrimidine: Bound to sugars, they form nucleosides that can combine with phosphate residues to nucleotides. Nucleotides have an outstanding role in the cell as energetic compounds (ADP, ATP and others) , co-factors (NAD, NADP) and components of nucleic acids.