C. References
III. The Iron and Magnesium Branches of the Porphyrin Biosynthetic Pathway
A. The Iron Branch of the Porphyrin Biosynthetic Pathway: Biosynthesis of Heme
Protoporphyrin IX is the point at which the heme and the Chl pathways diverge.
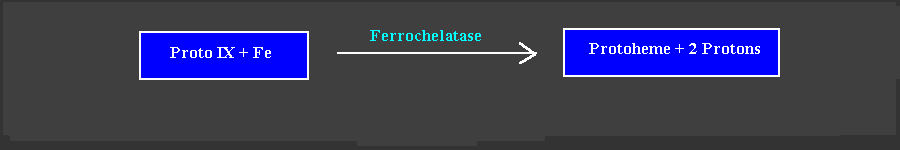
The terminal step in heme biosynthesis involves insertion of ferrous iron into Proto by ferrochelatase to yield protoheme IX (Goldberg et al 1956). Ferrochelatase was first purified from rat liver (Taketani and Tokunaga, 1981). Insertion of Fe++ into Proto is accompanied by the release of two protons from the pyrrole nitrogens. Mammalian ferrochelatase has a reported molecular weight of about 40,000. In plants the enzyme is found in the mitochondria and the plastids (Little and Jones, 1976). Specificity of the enzyme for Proto is not absolute, as the enzyme is able to handle a variety of porphyrin IX isomers, with substituents at the 2 and 4 positions of rings A and B, that are smaller in size than hydroxyethyl and are uncharged. In animal cells, conversion of Proto to protoheme takes place in the mitochondria. In Euglena, It has been reported that protoheme is formed in the mitochondria from ALA formed via the glycine-succcinate pathway, and in the plastid from ALA formed via the C5-pathway (Weinstein and Beale, 1983). In is not clear wether the same situation prevails in higher plants.
B. The Mg-Branch of the Porphyrin Biosynthetic Pathway
The role of Proto as the starting point of the Mg- branch of the porphyrin pathway and as an intermediate in the Chl biosynthetic pathways was based on the detection of Proto in X-ray Chlorella mutants inhibited in their capacity to form Chl (Granick, 1948). It was conjectured that since the mutants had lost the ability to form Chl but accumulated Proto, the latter was a logical precursor of Chl. The unambiguous role of Proto as a precursor of all Mg-porphyrins and phorbins including Chl was established (a) by conversion of exogenous Proto to Mg-Proto monomethyl ester (Mpe) by Rhodopseudomonas spheroides in the presence of ATP and Mg (Gorchein, 1972), and (b) by conversion of exogenous14C- and unlabeled-Proto to Pchlide a [the immediate precursor of chlorophyllide (Chlide) a] in organello (Mattheis and Rebeiz, 1977a), using a cell-free system capable of the conversion of 14C-ALA to 14C- Pchlide a, 14C-Pchlide ester a and 14C-Chl a and b (Rebeiz, and Castelfranco, 1971a, Rebeiz and Castelfranco, 1971b), and capable of the net conversion of exogenous ALA to Mg-Protoporphyrins and Pchlide a (Rebeiz, et al, 1975).
References
- Goldberg, A. Ashenbrucker, M., Cartwright, G. E., and Wintrobe, M. M. (1956). Studies on the biosynthesis of heme in vitro by avian erythrocytes. Blood, 11:821-833.
- Taketani, S., and Tokunaga, R. (1981). Rat liver ferrochelatase. Purification, properties and stimulation by fatty acids. J. Biol. Chem. 256:2748-12753.
- Little, H. N. and Jones, O. T. G. (1976). The subcellular localization and properties of the ferrochelatase of etiolated barley. Biochem. J. 156: 309-314.
- Weinstein. J. D., and Beale, S, I. (1983). Separate physiological roles and subcellular compartments for two tetrapyrrole biosynthetic pathways in Euglena gracilis J. Biol. Chem. 258:6799-6807.
- Granick, S. (1948). Protoporphyrin 9 as a precursor of chlorophyll. J. Biol. Chem. 172:717-727.
- Gorchein, A. (1972). Magnesium protoporphyrin chelatase activity in Rhodopseudomonas spheroides. Studies with whole cells. Biochem. J. 127:97-106.
- Mattheis, J. R. and C. A. Rebeiz. (1977a). Chloroplast biogenesis: Net synthesis of protochlorophyllide from protoporphyrin IX by developing chloroplasts. J. Biol. Chem. 252:8347-8349.
- Rebeiz, C. A., and P. A. Castelfranco. (1971a). Protochlorophyll biosynthesis in a cell-free system from higher plants. Plant Physiol. 47:24-32.
- Rebeiz, C. A., and P. A. Castelfranco. (1971b). Chlorophyll biosynthesis in a cell-free system from higher plants. Plant Physiol. 47:33-37
- Rebeiz, C. A., B. B. Smith, J. R. Mattheis, C. C. Rebeiz and D. F. Dayton (1975). Chloroplast biogenesis. Biosynthesis and accumulation of protochlorophyll by isolated etioplasts and developing chloroplasts. Arch. Biochem. Biophys. 171:549-567.
Go Back to Main Menu