C. References
IX. The Fully Esterified Chl a Biosynthetic Routes: Reactions between Mg-Protoporphyrin diester (Mpde) and Chl a
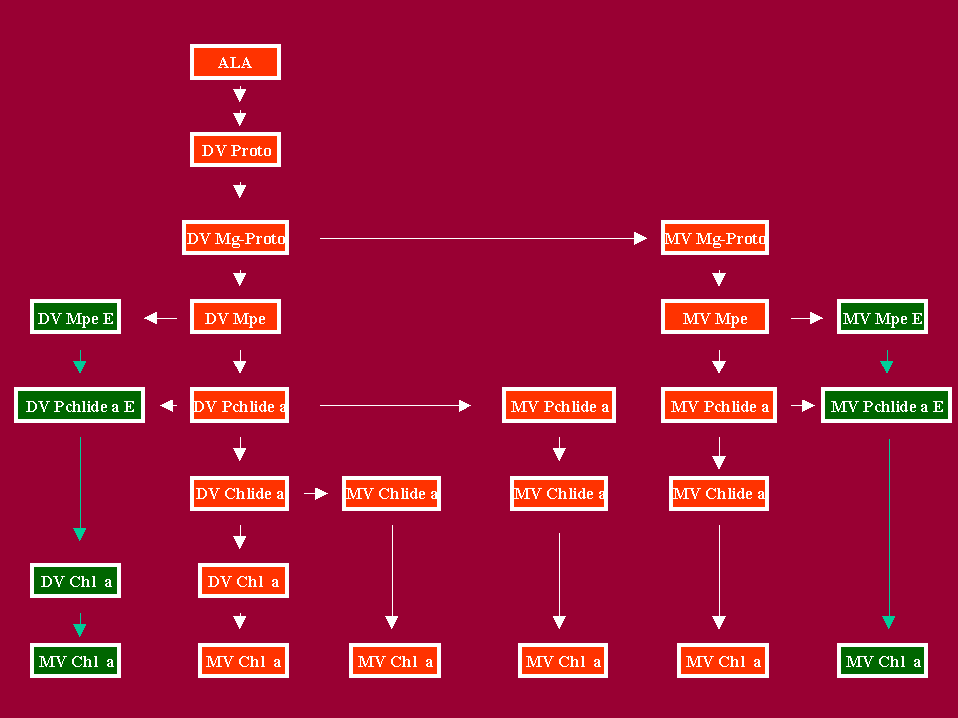
Fig. 3: Reactions of the DV and MV Carboxylic and Fully Esterified Chl a Biosynthetic Routes Between DV Mg-Proto and Chl a
The reactions between ALA and DV Proto are shown in more detail in Fig. 1. The various Chl a carboxylic biosynthetic routes are discussed in sections I to VIII. DV= divinyl; MV = monovinyl; Mg-Proto = Mg-protoporphyrin IX; Mpe = Mg-Proto monomethyl ester; Mpde = Mg-Proto diester; Pchlide a= protochlorophyllide a; Pchlide a E = protochlorophyllide a ester; Chlide a = Chlorophyllide a; Chl a = chlorophyll a; 4VMPR = [4-vinyl] Mg-Protoporphyrin IX reductase; 4VPR = [4-vinyl] protochlorophyllide a reductase; 4VCR = [4-vinyl] chlorophyllide a reductase. Arrows joining the DV and MV branches refer to reactions catalyzed by [4-vinyl] reductases. Arrows joining the carboxylic and fully esterified branches refer to reactions catalyzed by porphyrin ester synthetases. Adapted from Rebeiz et al, 1983.
The fully esterified Chl a pathway is populated by tetrapyrroles with a methyl propionate residue at position 6 of the macrocycle and a propionic acid residue at position 7 which is esterified with a long chain fatty alcohol (Rebeiz et al. 1983). This pathway deals with the least understood phase of the intermediary metabolism of Chl a . In what follows a brief account of what is known about this pathway is presented.
A. Mpde
1. Detection of Mpde
A fully esterified Mg-Proto diester (Mpde) pool was first detected in etiolated cucumber cotyledons incubated overnight with ALA and 2,2'-dipyridyl (Dpy) in darkness (McCarthy et al, 1981). The novel pool exhibited the chromatographic properties of a fully esterified metalloporphyrin and the spectrofluorometric properties of Mg-protoporphyrin IX. Chemical derivatization coupled to spectrofluorometric and chromatographic analysis identified it as a Mg-Proto diester. High-pressure liquid chromatographic analysis indicated that the Mpde pool was heterogeneous and consisted of three fully esterified Mg-Protos. Gas-chromatographic /mass spectroscopic analysis of the saponified alcohol fraction of the heterogeneous Mpde pool revealed that the latter consisted of three major long-chain alcohols, none of which was identifiable with known isoprenoids such as geraniol, farnesol or phytol. Mpde was also detected in dark-grown Euglena gracilis and in etiolated cucumber cotyledons incubated in darkness with ALA, in the absence of added Dpy. It was suggested that Mpde may be a metabolic precursor of the fully esterified, heterogeneous, Pchlide a ester pool (McCarthy et al, 1981).2. The DV/MV Nature of the Mpde pool
In addition to the heterogeneity of the long chain alcohols esterifying the propionic acid residue at position 7 of the macrocycle (McCarty et al, 1981), the Mpde pool exhibited a well pronounced DV-MV heterogeneity (Belanger and Rebeiz, 1982). For example in etiolated cucumber cotyledons incubated in darkness with ALA and Dpy, as well as in dark-grown Euglena gracilis, the Mpde pool consisted of DV and MV Mpde components. The biosynthetic origin of DV and MV Mpde is not presently clear, and is tentatively assigned to DV and MV Mpe esterification (Fig. 3)
C. Pchlide a ester
Fischer and Oestreicher (1940) synthesized the phytyl ester of Pchlide a and showed that it differed from MV Chl a in having two fewer hydrogens at position 7 and 8 of the macrocycle. They named this molecule protochlorophyll. Because of the structural similarity between Pchlide a phytyl ester and Chl a the erroneous notion evolved that Pchlide a phytyl ester was the major immediate photoprecursor of Chl a (Smith, 1948; Koski, 1950). When Granick, (1950) isolated and identified Pchlide a from an X-ray Chlorella mutant inhibited in its capability to form Chl, he considered it to be the immediate precursor of Pchlide a ester. The biological function of Pchlide a as the immediate precursor of chlorophyllide (Chlide) a was not fully understood till seven years later (Wolff and Price, 1957).
1. Nature of the Long Chain Fatty Alcohol at Position 7 of the Pchlide a ester Macrocycle
As early as 1958 various researchers started questioning the assumed phytol nature of the long chain fatty alcohol that esterified the propionic acid residue at position 7 of the Pchlide a ester macrocycle (Rebeiz and Castelfranco, 1973). For example gas chromatographic analysis of the hydrolyzed fatty alcohol fraction of Pchlide a ester of etiolated cucumber cotyledons failed to detect any phytol (Rebeiz and Castelfranco, 1973; McCarthy et al, 1981). On the other hand, the Pchlide a ester of etiolated barley leaves was shown to contain geranylgeraniol (GG) instead of phytol (Liljenberg, 1974). The inner seed coat of Cucurbitaceae, a rich source of Pchlide a ester was shown to contain a large number of Pchlide a esters esterified with different long chain alcohols. The latter consisted of farnesol and all possible C20 alcohols including GG and phytol (Shioi and Sasa, 1982). Roots of etiolated wheat, accumulated large amounts of MV Pchlide a esters and lesser amounts of MV Pchlide a. The alcohol moieties of the four accumulated Pchlide a esters consisted of GG, dihydrogeranylgraniol (GHGG), tetrahydrogeranylgraniol (THGG) and phytol (Mc Ewen and Lindsten, 1992).2. DV-MV Nature of the Pchlide a ester pool
The Occurrence of DV Pchlide a ester in higher plants was first reported in the inner seed coat of Cucurbita pepo (pumpkin) (Jones, 1966), and was confirmed by Houssier and Sauer (1969). Search for the occurrence of DV Pchlide a ester in other higher plant tissues was however unsuccessful till 11 years later (Belanger and Rebeiz, 1980). Using sensitive spectrofluorometric techniques and chromatography on thin layers of polyethylene, Belanger and Rebeiz were able to show that etiolated cucumber cotyledons (a dark DV-light DV plant tissue) incubated in darkness with delta-aminolevulinic acid (ALA), accumulated mainly MV Pchlide a ester and very small amounts of DV Pchlide a ester. The two Pchlide a esters were separated by chromatography on thin layers of polyethylene and were characterized by their fluorescence emission and excitation spectra at room temperature and 77 K. However, these studies were not extended with rigor to MV plant species such as wheat corn and barley. In other words, it is not certain at this stage whether small amounts of DV Pchlide a ester also occur in DMV-LMV and DMV-LDV plant species.
3. Biosynthesis of Pchlide a ester
Because of the structural similarity between the Pchlide a and Pchlide a ester macrocycles it was convenient to propose that Pchlide a was the immediate precursor of Pchlide a ester (Granick, 1950). As early as 1970, In-vivo precursor-product relationship studies between the biosynthesis of 14C-Pchlide a and 14C-Pchlide a ester failed to establish precursor-product relationship between these two metabolites. instead, the studies indicated that Pchlide a and Pchlide a ester were most probably formed from a common precursor (Rebeiz et al, 1970). This was followed by in-vitro investigations which also failed to establish precursor product relationships between Pchlide a and Pchlide a ester (Ellsworth and Nowak, 1973, Mattheis and Rebeiz, 1977). More rigorous in-vitro precursor-product relationship studies between Pchlide a and Pchlide a ester were carried out by McCarthy et al (1982). Comparison of the ratio of 14C-ALA and various 14C-tetrapyrrole substrates into 14C-Pchlide a and 14C-Pchlide a ester, allowed the determination of which exogenous 14C-tetrapyrrole substrate was the most likely common precursor of Pchlide a and Pchlide a ester. On the basis of these studies, it was proposed that Pchlide a is formed via an acidic (mono/dicarboxylic) biosynthetic route while Pchlide a ester is formed via a fully esterified route. It was also proposed that the two routes are weakly linked at the level of Proto, Mg-Proto, Mpe and Pchlide a by porphyrin ester synthetases (McCarthy et al, 1982). Because of the DV-MV nature of the detected Mpde and Pchlide a ester pools, the fully esterified route is depicted as being split into DV and MV routes (Figure 3). It should be pointed out however, that this hypothesis is based on detection of putative intermediates and has not yet been confirmed by demonstration of precursor product relationships between the Mpde and Pchlide a ester components of the two putative routes.4. Function of Pchlide a ester
Biosynthetically and functionally, the Pchlide a ester pool is the least understood pool of the Chl biosynthetic pathway. The full extend of its biological function is still unclear and is surrounded by controversy. At one time, on the basis of its structural similarity to MV Chl a, it was assumed to be the immediate major photoprecursor of MV Chl a (Smith, 1948; Koski, 1950; Granick, 1950). That hypothesis lost its appeal when Wolff and Price (1957) demonstrated that the major immediate precursors of MV Chl a were MV Pchlide a and MV Chlide a. Thus for a while Pchlide a ester floated as a tetrapyrrole pool without any defined function. However by 1973, several laboratories had reported that Pchlide a ester was probably partially photoconvertible to Chl a (for a review of this early work, see Rebeiz and Castelfranco, 1973). Some post-1973 work about the photoconvertibility of Pchlide a ester to Chl a is described below.a. Photoconversion of MV Pchlide a ester to MV Chl a
Addition of two trans-hydrogens across the 7-8 position of the MV Pchlide a ester macrocycle would result in the conversion of MV Pchlide a ester to MV Chl a. Several laboratories have reported such a reaction in higher plants (Rebeiz and Castelfranco, 1973; Liljenberg, 1974; Lancer et al, 1976; Belanger and Rebeiz, 1980), and lower plants (Sasa and Sugahara, 1976; Kotzabasis et al, 1989). Since other researchers have not been able to detect the photoconversion of Pchlide a ester, Rudiger and Schoch (1991) suggested that such discrepancies may be due to age of seedlings or the very rapid esterification of Chlide a to Chl a during the light treatment. The latter possibility is unlikely as the photoconversion of Pchlide a ester has been also observed at temperatures of -15 to 2 Celsius (Rebeiz and Castelfranco, 1973, Liljenberg, 1974). In our opinion, failure to observe the photoconversion of Pchlide a ester to Chl a stems from two considerations: (a) The photoconversion is only partial and very small amounts of Chl a are formed, (b) detection of such small amounts of Chl a depend a great deal on the sensitivity of the instrumentation in use. We have recently reexamined the photoconversion of Pchlide a ester to Chl a in isolated cucumber etioplasts. Reaction products were determined by HPLC coupled to very sensitive spectrofluorometric detection. Our results reconfirmed the partial photoconvertibility of the Pchlide a ester (Adra and Rebeiz, unpublished).
b. Photoconversion of DV Pchlide a ester to DV Chl a
In Fig. 3, the photoconversion of DV Pchlide a ester to DV Chl a is assigned to a fully esterified DV Chl a biosynthetic route. This assignment is based on the detection of DV Chl a formation immediately following a 47 ms actinic white light treatment of etiolated cucumber cotyledons, at room temperature (Belanger and Rebeiz, 1980). It was assumed that the small amounts of DV Chl a were as a consequence of the photoconversion of small amounts of DV Pchlide a ester. It has since come to our attention, that at room temperature, in-vivo, conversion of newly formed DV Chlide a to DV Chl a is extremely rapid (see conversion of DV Chlide a to DV Chl a). As a consequence the possible photoconversion of DV Pchlide a ester to DV Chl a should be confirmed with isolated plastids at subzero temperatures.
References
- Rebeiz, C. A., S. M. Wu, S. M., Kuhadja M., Daniell, H. and Perkins E. J. (1983). Chlorophyll biosynthetic routes and chlorophyll a chemical heterogeneity in plants. Mol. Cell. Biochem. 58:97-125.
- McCarthy, S. A., Belanger F. C., and Rebeiz, C. A. (1981). Chloroplast biogenesis: Detection of a magnesium protoporphyrin diester pool in plants. Biochemistry 20: 5080-5087.
- Belanger, F. C. and Rebeiz, C. A. (1982). Chloroplast Biogenesis: Detection of monovinyl magnesium protoporphyrin monoester and other monovinyl magnesium porphyrins in higher plants. J. Biol. Chem. 257: 1360-1371.
- Fischer H. and Oestreicher, A. (1940). Z. Physiol. Chem. 262 : 243.
- Smith, J. H. C. (1948). Protochlorophyll, precursor of chlorophyll. Arch. Biochem. 19: 449-454.
- Koski, V. M. (1950). Chlorophyll formation in seedlings of Zea Mays L. Arch. Biochem. 29: 339-343.
- Granick, S. (1950). Magnesium vinyl pheoporphyrin a5, another intermediate in the biological synthesis of chlorophyll. J. Biol. Chem. 183: 713-730.
- Wolff, J. B., and Price, L. (1957). Terminal steps of chlorophyll a biosynthesis in higher plants. Arch. Biochem. Biophys. 72: 293-301.
- Rebeiz, C. A. and Castelfranco, P. A. (1973). Protochlorophyll and chlorophyll biosynthesis in cell-free systems from higher plants. Ann. Rev. Plant Physiol. 24: 129-172.
- Liljenberg, C. (1974). Characterization and properties of a protochlorophyllide ester in leaves of dark grown barley with geranylgeraniol as esterifying alcohol. Physiol. Plant. 32: 208- 213.
- Shioi, Y. and Sasa, T. (1982). Separation of protochlorophylls esterified with different alcohols from inner seed coats of three cucurbitaceae. Plant &Cell Physiol. 23: 1315-1321.
- Mc Ewen, B. and Lindsten, A. (1992). Characterization of protochlorophyllide and protochlorophyllide ester in roots of dark-grown plants. Physiol. Plant. 84: 343-350.
- Jones, O. T. G. (1966). A protein-protochlorophyll complex obtained from inner seed coats of Cucurbita pepo. Biochem. J. 101: 153-160.
- Houssier, C. and Sauer, K. (1969). Optical properties of the protochlorphyll pigments. I. Isolation, characterization and infrared spectra. Biochem. Biophys. Acta. 172: 476-491.
- Belanger, F. C. and Rebeiz, C. A. (1980). Chloroplast Biogenesis: Detection of divinylprotochlorophyllide ester in higher plants. J. Biol. Chem. 19: 4875-4883.
- Rebeiz, C. A., Yaghi, M., Abou-Haidar, M. and Castelfranco, P. A. (1970). Protochlorophyll biosynthesis in cucumber (Cucumis sativus L.) cotyledons. Plant Physiol. 46: 57-63.
- Ellsworth, R. K. and Nowak, C. A. (1973). The inability of crude homogenates of etiolated wheat seedlings containing protochlorophyllase to convert 14C-protochlorophyllide to 14C-protochlorophyll. Photosynthetica. 7: 246-251.
- Mattheis, J. R. and Rebeiz, C. A. Chloroplast biogenesis XVII. Metabolism of protochlorophyllide and protochlorophyllide ester in developing chloroplasts. Arch. Biochem. Biophys. 184: 189-196.
- McCarthy, S. A., Mattheis, J. R. and Rebeiz, C. A. Chloroplast Biogenesis: Biosynthesis of protochlorophyll(ide) via acidic and fully esterified biosynthetic branches in higher plants. Biochemistry 21: 242-247.
- Lancer H. A., Cohen, C. E., and Schiff, J. A. (1976). Changing ratios of phototransformable protochlorophyll and protochlorophyllide of bean seedlings developing in the dark. Plant Physiol. 57: 369-374.
- Belanger, F. C., and Rebeiz, C. A. (1980). Chloroplast Biogenesis 30. Chlorophyll(ide) (E459 F675) and chlorophyll(ide) (E449 F675). The first detectable products of divinyl and monovinyl protochlorophyll photoreduction. Plant Sci. Lett. 18: 343-350.
- Sasa, T. and Sugahara, K. (1976). Photoconversion of protochlorphyll to chlorophyll a in a mutant of Chlorella regularis. Plant & Cell Physiol. 17: 273-279.
- Kotzabasis, K. Schuring, M-P. and Senger, H. (1989). Occurrence of protochlorophyll and its phototransformation in mutant C-2A' of Scenedesmus obliquus. Physiol. Plant. 75: 221-226.
- Rudiger, W. and Schoch, S. (1991). The last steps of chlorophyll biosynthesis. In: Chlorophylls (H. Scheer, ed.) pp 451-464, Academic Press, New York.