 |  | 
|
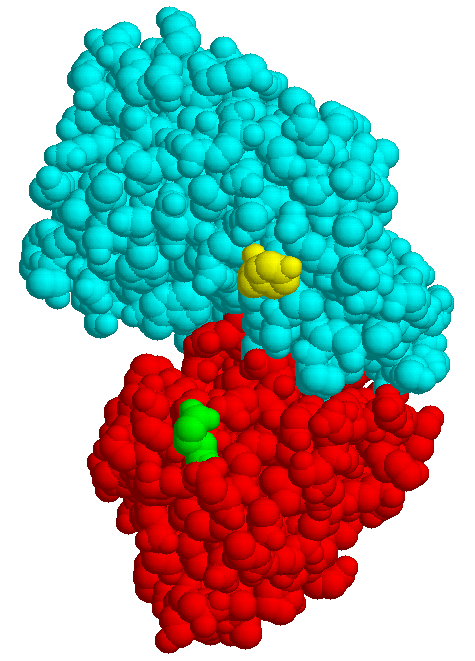
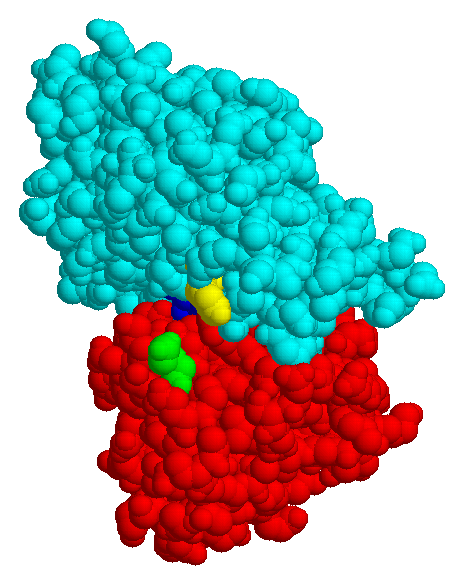
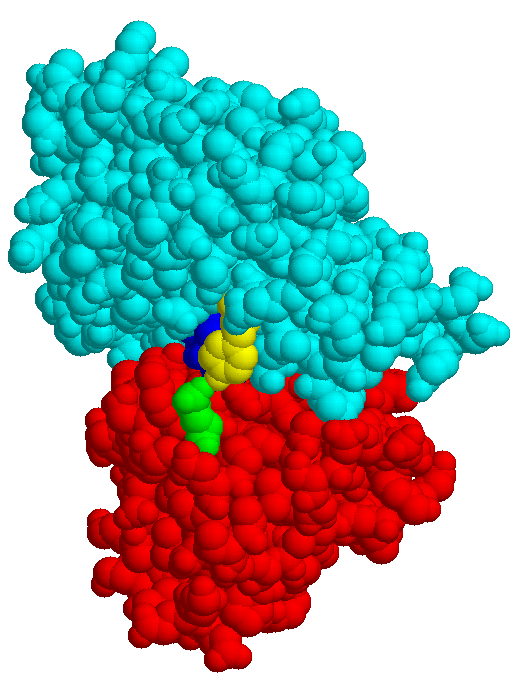
| MBP without substrate Glu45 Tyr 341 domain I domain II |
MBP with maltose | MBP with maltotriose |

|
Bacteriohodopsin from Halobacterium halobium
red: retinal |
Depending on the source of energy primary transport is differentiated from secondary transport. Primary transport uses energy directly: light or chemical energy is converted to electrochemical energy as electrochemical potential of the substances to be transported. This category comprises photosynthetic electron transport, light driven ion pumps, redoxenergy dependent respiratory chains, transport ATPases and sodium pumps utilizing decarboxylation energy.
In secondary transport the electrochemical energy originates from the electrochemical potential of another substance that is used up in symport or antiport.
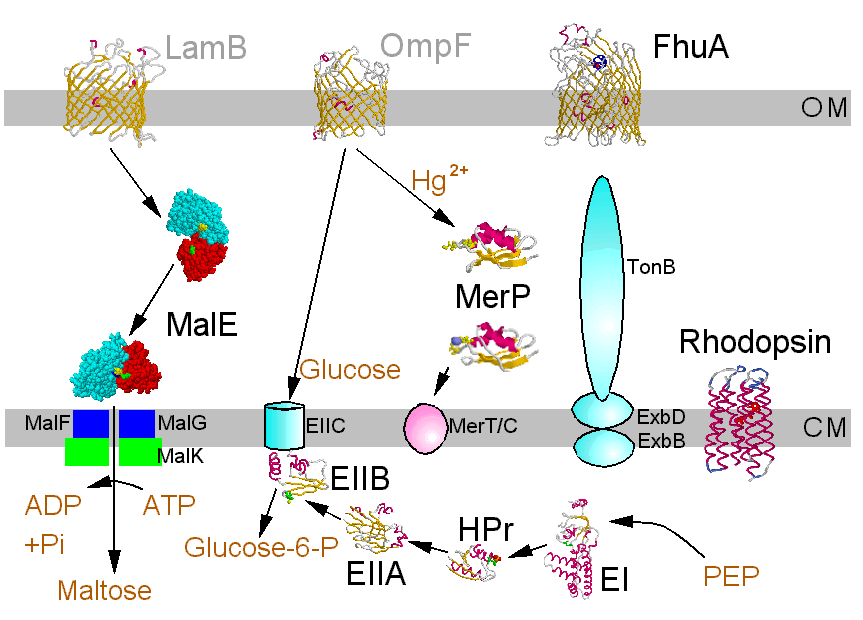
Normally a molecule passes the membrane unchanged. However, in group translocation there is a chemical modification. This method is used by bacteria in the phosphoenolpyruvate:sugar phosphotransferase system (PTS).
Transport systems for nutrients are directed into the cell; all prokaryotic as well as eukaryotic cells contain additionally systems with a reverse transport direction to get rid of toxic substances. These systems may severely impair therapeutic efforts (multidrug efflux systems). Special transport systems protect producers of antibiotics or toxins against their own poisons.
The structures of few transport systems are known at atomic resolution. Among these are rhodopsins, some components of the PTS, and binding proteins used by bacteria in conjunction with transport ATPases. Binding proteins are found anchored to the cytoplasmic membrane of Gram-positive bacteria or free in the periplasm of Gram-negative bacteria (binding protein dependent transporters), they belong to the superfamily of ABC (ATP bindig cassette) transporters. Sugar specific binding proteins may change their conformation considerably upon binding a ligand, as the maltose binding protein of E. coli:
 |  | 
|
| MBP without substrate Glu45 Tyr 341 domain I domain II |
MBP with maltose | MBP with maltotriose |
Examples shown here are the binding proteins involved with the transport of maltodextrins and mercury, for proton transport a bacteriorhodopsin and components of the glucose PTS from E. coli. As an exemption from the rule there is FhuA, which is an energy dependent transport system located in the outer membrane of E. coli specific for siderophor bound iron and misused for antibiotics, phage DNA and colicins. Click the structure icons for details.