
Botany online 1996-2004. No further update, only historical document of botanical science!
In comparison to procaryotes, eucaryotic cells are large. Their surface to volume ratio is less advantageous. They are, moreover, characterized by extensive intracellular membrane systems. The outer membrane (the plasmalemma) is very flexible. It can adapt to extern unevenness, it can form evaginations, and it can give off vesicles either to the inside or to the outside. Eucaryotic cells are with few exceptions aerobic. Compelling evidence for the assumption, that this has not always been the case and that primitive eucaryotic cells have originally been anaerobic exists.
The increase in size required an increase in metabolism. Eucaryotic cells must have developed in environments that were rich in nutriments, since the photosynthetic activity of blue-green algae produced not only large quantities of free oxygen, but also stoecheometric amounts of fixed carbon and made thus for an accumulation of biomass unknown to have existed ever before. Most likely, primitive eucaryotic cells profited of it. Heterotrophic cells used the supply of nutriments and gained size.
How else do eucaryotes differ from procaryotes?
Eucaryotic cells contain certain structure-forming molecular complexes (a cytosceleton) responsible for movements: actin and myosin, their accompanying regulators, and tubulin.
Their genetic information is located on several molecules of DNA (chromosomal equivalents) and is stored in a functional unit enclosed by a membrane, the nucleus.
Mitosis, a mechanism for the distribution of chromosomes and chromosome equivalents developed with the help of tubulin.
The ability of large and cell wall-less eucaryotic cells to take up organic material led also to their taking up smaller cells, a process called phagocytosis.
Phagocytosis may in some cases not have resulted in the digestion of the smaller cells, but in a symbiosis with them, a state also known as endosymbiosis. According to the today mostly accepted endosymbiontic theory, primitive amoeboid eucaryotic cells took up aerobic bacteria as symbionts. Both partners became a unit that was then able to use the decreasing amounts of nutriments far more efficiently. These now aerobic eucaryotes kept their heterotrophic life-style.
A few of the aerobic eucaryotes started a symbiosis with blue-green algae or procaryotes similar to blue-green algae and became thus the precursors of plant cells characterized by their photoautotrophic feeding habit.
Eucaryotic cells acquired the ability to differentiate, to divide function, and to develop into multicellular organisms.

Eucaryotes can be grouped into four kingdoms:
- protista (usually single-celled species)
- plants (plantae)
- fungi (fungi), and
- animals (animalia).
Procaryotes belong to two further kingdoms, eubacteria and archaebacteria, as well as to the evolutionary line leading to the eucaryotes
(according to R. H. WHITTAKER).
There is no doubt that plants fungi and animals developed from ancestors that belonged to the protists. But how did the features listed under (1.) - (7.) came to being? How did their evolution look like?
On 1: Actin, myosin and tubulin occur neither in eubacteria nor in archaebacteria. These three proteins cannot be traced back to a common ancestor, since their differences outline their similarities by far.
© James A. Sullivan: http://www.cellsalive.com
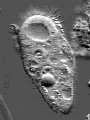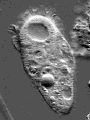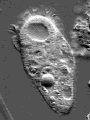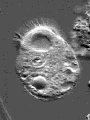
Actin is one of the most common proteins of animal and many protist cells, of slime moulds, and plant cells. It is a component of contractile elements called microfilaments. It participates in amoeboid movements, and has accordingly a very likely role in phagocytosis. Since amoebas depend on an extern supply of nutriments, the ability to move allowed them to go hunting.
Tubulin is a protein that can just like actin form aggregates with microtubuli, contractile elements, too. It is essential for two types of movements:
The movements of chromosomes during mitosis. The nuclear spindle consists mainly of microtubules.
The movements of cilia and flagelli. The flagelli and cilia of all eucaryotic cells are organized according to a uniform 9 + 2 scheme. In contrast to the flagelli of bacteria, eucaryotic flagelli and cilia are intracellular structures. The bundles of microtubuli are , just like the rest of the cell's content, surrounded by a continuous membrane, the plasmalemma. Movement of flagelli occurs only, if energy in the form of ATP is supplied.
On 2. The nucleus that is surrounded by a double-membrane (a coat), must have developed rather early during evolution as no eucaryotic cells without this type of nucleus are known to exist. The DNA of nearly all eucaryotes is always associated with basic proteins, the histones. The association has a high adaptive value as expressed by the fact that four of the five histones (especially histone IV) have hardly changed during the diversification of plants and animals. Some ciliates, like Tetrahymena, contain histone IV that differs from its plant and animal equivalent in 22 percent of its amino acid residues. This could mean that the interaction between histones and DNA is not yet fully optimized at the evolutionary level of the protists.
On 3. The existence of a nucleus is not the same as the existence of a mitosis. In the case of many protists and some fungi, like yeast, no chromosomes can be recognized. During the division of the nucleus and the cell, a seemingly confused tangle of threads appears. The existence of coupling groups seems to have caused a grouping of the genetic material into different structural units. The situation is similar in some chloroplast-containing flagellates like Euglena.
In numerous dinoflagellates, several accumulations of fibrillary looking structures occur during the division of both the cell and the nucleus. They could in a functional, but not in a structural sense be called chromosomes. No spindle apparatus is formed. The separation of the genetic material occurs within the nuclear envelope. In other dinoflagellates again, first signs of a spindle apparatus organization outside of the nucleus have been observed. The spindle fibres, nevertheless, do not make contact with the DNA as the nuclear envelope remains intact and between these two molecular aggregates in these cases, too. In a third group, clearly demarcated areas can be recognized within the membrane in electron-molecular studies that seem to act as specific adaptors between DNA and microtubuli. They may be precursors of the later site of spindle attachment, the centromer, but definite proof of homology does not yet exist (D. F. KUBAL, 1975).
In the case of mitosis, what has been said earlier about valuable structures applies here, too: after a mechanism had evolved and was perfected, it remained and was not altered any further. Quite a number of protists, where this state has already been reached, are known. It is striking, that protists contain a large numbers of chromosomes, several hundred actually, while the genetic information of multicellular organisms is concentrated on usually far less than fifty chromosomes. It seems to be simpler to distribute a small number of units regularly and without mistakes onto daughter cells than a large one.
On 4-6. The endosymbiontic theory became more and more likely during the last years as the number of correspondences between mitochondria and chloroplasts became larger and larger the more their details and that of their potential precursors were studied. The probability that these two organelles developed de novo in parallel to procaryotes seems by now to be pretty small.
At first glance, the argumentation seems not necessarily conclusive, but the more became known about the organization and variability of genetic material within cells the easier it became to clarify seeming contradictions.
What is to be said for mitochondria having evolved from bacteria? Mitochondria are semi-autonomously working organelles. They contain DNA and a machinery for protein synthesis. Their ribosomes belong to the 70 S type and chloramphenicol inhibits their protein synthesis. In contrast, the ribosomes found in the cytosol of the eucaryotic host are all of the 80 S type. The host’s protein synthesis is inhibited by cycloheximid, but not by chloramphenicol.
The DNA of mitochondria is circular just like in bacteria, though it has to be said that almost all DNA molecules of this size are circular independent of their origin. It is not associated with histones. Its information content is far smaller than that of any species of bacteria. It is assumed that the rest of the information got lost during the course of evolution as it was no longer required for the survival of the symbiont. Meanwhile, it is known that parts of the originally mitochondrial DNA were incorporated into the host’s nuclear genome.
Mitochondria have no cell wall. Their inner membrane contains certain lipids that are only found in mitochondria and bacteria. It is assumed that the outer mitochondrial membrane is supplied by the host cell and that it is homologous to a phagocytosis membrane. Its lipid composition does not differ from that of other host cell membranes.
In bacteria and in the inner mitochondrial membrane, the enzymes of the respiratory chain have the same spatial arrangement. The essential enzymes of the bacterial and the mitochondrial respiratory chain are homologous. The highest degree of correspondence exists between the respiratory chain of mitochondria and rhodospirills.
Very few recent eucaryotic cells have no mitochondria. Among them is the ameoba Pelomyxa palustris. It lives in symbiosis with aerobic, cell-wall containing bacteria. This is an important evidence for the coming into being of endosymbionts under natural conditions.
Just like mitochondria can be traced back to originally endosymbiontic bacteria, chloroplasts are homologous to blue-green-algae-like prokaryotes. Plastid-containing organisms belong always to the plant kingdom. The position of Euglena, a chloroplast-containing group of the euglenophyta is somewhat doubtful as – in contrast to real plants – plastid-free mutants of usually plastid-containing species occur. Even species lacking plastids completely exist. What kinds of similarities exist between chloroplasts and the plastids that stems from them and blue-green algae or their precursors?
The degree of homology between the rRNA of chloroplasts and that of recent blue-green algae is very high and is expressed in nearly identical secondary structures. The DNA of chloroplasts, nevertheless, contains introns while that of blue-green algae does not.
Chloroplasts and blue-green algae contain the photosystems I and II. The single reactions of the photosystems of chloroplasts and blue-green algae do mostly correspond, even though the chloroplasts of green algae contain chlorophyll a and b, while recent blue-green algae contain no chlorophyll b. Recent blue-green algae do however contain chlorophyll a and phycobilins (phycocyanin and phycoerythrin), a combination that is also known from the plastids of red algae.
The large and the small ribosomal subunits of chloroplasts and bacteria complement each other. Reconstitution experiments showed that single ribosomal proteins of bacteria substitute for that of chloroplasts.
The DNA of chloroplasts contains just like the DNA of mitochondria relatively little genetic information. Many of the proteins identified and required in chloroplasts are encoded by the nucleus. A comparison between the ATP-synthetases of mitochondria of varying origins and that of chloroplasts shows that the enzymes of both organelles share many similarities. ATP-synthetase consists of several polypeptide chains. Its corresponding genes are partly located in the nucleus, partly in the organelles themselves. The results given as a scheme show that it is not always the same set of genes that is located in the nucleus or the organelles, respectively.
In some algae and euglenophyta, so-called cyanelles have been found. Cyanelles are inclusions, intermediates between blue-green algae and chloroplasts. The cyanelles of the euglenophyte Cyanophora paradoxa, for example, contain only 5 – 10 percent of the blue-green algae’s genome. Cyanelles are surrounded by a murein-containing wall and live in intracellular vesicles. The genes of the large and the small subunit of ribulose-1.5- bisphosphate carboxylase are located in the cyanelle genome. In green plants, the small subunit is encoded by the nucleus, the large subunit by the plastid. Cyanelles and host cells depend on each other, none of them can be cultivated alone, i.e. their integration is already far advanced, but has not progressed as far as that of chloroplasts and their host cells.
In 1976, R. A. LEWIN discovered a species called Prochloron that is similar to blue-green algae and lives endosymbiontically in the cells of a sponge. It contains chlorophyll a and b and its thylacoids are stacked in the way that is typical for chloroplasts. This led to the assumption that blue-green algae resembling Prochloron were more likely to be the predecessors of chloroplasts than any other recent species. Pochloron, nevertheless, is too specialized in many features, so that this assumption had to be discarded for the time being.
In 1986, a Dutch group of researchers (T. BURGER-WIERSMA, 1991) described a further species similar to Prochloron called Prochlorothrix hollandica. It was found in a flat, eutrophic lake, the Loosdrechtsee, were it occurred in masses in 1984. The cells are long and their pigment composition resembles that of the chloroplasts of green plants (T.BURGER-WIERSMA, 1991).
The large differences in the pigmentation and the ultrastructure of the chloroplasts of red algae, brown algae, diatoms, and green plants make a single incident as the initiation of endosymbiosis unlikely. Moreover, it looks as if the single plant groups acquired their plastids separately. The plant kingdom does thus seem to be of polyphyletic origin. The endosymbionts do most likely belong to different groups of blue-green algae (see also point 4).
The ATP-synthetase of bacteria and blue-green algae resembles that of organelles and consists of the same amount of subunits. This finding points at a procaryotic origin of the enzyme and makes it probable that during evolution, genes from the endosymbionts or the organelles derived from them migrated into the host cell’s nucleus and were incorporated into its genome. These results show, too, that the migrations of single genes have to be regarded as independent incidents.
In some coccal algae like Glaucosphaera vacuolaria, cyanelles with almost no or completely lacking cell walls occur. Some marine and chloroplast-containing diatoms, the bacillariophyceae, live in a facultative symbiosis with the thread-shaped blue-green algae Richelia. The symbiont is integrated into the host cell’s plasma. This latter example of an incomplete and reversible integration is good instance for the fact that endosymbioses can occur again and again. The other examples show that the single steps leading to the transformation of a procaryont into an organelle are separate and independent of each other. Cyanelles are thus good models for the in-detail analysis of the process of integration.
The arguments listed when discussing mitochondria (point 4) show that a regulated integration of the endosymbionts developed that led finally to both mitochondria and chloroplasts becoming indispensable for their host cells. Namely, no eucaryotic cells without organelles besides the already mentioned exceptions exist.
What effects does the co-operation between the single compartments of the cell – the plastids, mitochondria, cytosol, and the nucleus - have ?
Proteins like the ATP-synthethase that consist of several subunits and the production of which depends on the interaction of different genomes received much attention.
Rubisco, its full name is ribulose-1,5-bisphosphatecarboxylase or fraction-1-protein, is quantitatively the most common protein existing. It consists of small and large subunits, that are encoded by the nucleus (the small subunits) and the chloroplasts (the large subunits) in eucaryots. Rubisco occurs in blue-green algae, too, where all genes co-exist within one genome. In one type of cyanelle (Cyanophora paradoxa, see the paragraph above), both genes are located within the cyanelle’s genome. Isoelectric focussing, a variation of the electrophoretic separation, reveals an intraspecific variation of the small and the large subunit. The analysis of Rubisco’s variability within the genus Nicotiana opened up a new possibility to deduce phylogenetic connections between the single species.

Schematic Illustration of the Number and the Position of the Small and the Large Rubisco Subunits of Tobacco after their Separation by Isoelectric Focussing.
The Latin names of the Nicotiana-species are given (epitheta). It can be seen clearly that the pattern of the large subunit is the same in all Australian species and in some species of the western hemisphere. The rest of the other species gathers into three more groups: A, B, and C. The variability within the small subunit is far larger than that of the large subunit (according to K. CHEN; S. JOHAL, S. G. WILDMAN, 1976). See the text for more information. These data can be used to draw up a dendrogramm.
The large subunit shows less variation than the small one. This may be caused by the plastids’ high degree of polyploidy. Namely, in every cell exist many plastids and each of them has several equal molecules of DNA. The probability that one mutant catches on under these circumstances is far less likely than in a diploid nuclear genome.
The small subunit, too, is usually presented within a gel by more than two bandings. This is quite logical as the gene exists in tow different alleles. The cause for this are isoenzymes, i.e. products of different genes that came into being as a consequence of gene duplication or (allo-) polyploidy. The latter is characteristic of many Nicotiana-species. Nicotiana rustica (n= 24) developed from a hybridization of Nicotiana undulata (n= 12) x Nicotiana paniculata (n= 12), while Nicotiana arentsii (n= 24) is a hybridization product of Nicotiana wigandioides (n= 12) x Nicotiana undulata (n= 12). The hybrids contain the gene products, in this case the small subunit of Rubisco, of both parental species. Both are expressed in equal amounts. The large subunit is encoded by the chloroplast genome and as a consequence, only the genotype of the female parent can be found in the hybrid.
This method allowed to furnish the proof that the species Nicotiana tabacum (n= 24) is an amphidiploid hybrid of the maternal species Nicotiana sylvestris and the paternal species Nicotiana tomentosiformis as it contains the same type of large subunits as Nicotiana sylvestris. These subunits can only have been passed on to the new species Nicotiana tabacum by the maternal plasma.
An intraspecific variation of Rubisco may overlie its interspecific variation. Nicotiana suaveolens, for example, has a highly polymorphic phenotype. The large subunit is the same in all populations while the small one varies. Hybridization experiments showed that it is an alloenzyme.
Allopolyploidy is far spread in the genus Nicotiana, so that a dendrogramm based on the alloenzyme data should not be mixed up with a phylogenetic tree of the genus Nicotiana.
n order to safeguard statements about phylogenetic relations, morphological, caryological, and protein biochemical data have to be brought into line. This has already been done successfully in the case of the genera Brassica, Triticum, and Solanum.