Botany online 1996-2004. No further update, only historical document of botanical science!
Like all the groups reviewed until now, this one does also consist of a large number of molecules of heterogeneous structure. Their common feature is the presence of at least one hydroxyl-substituted aromatic ring system.
Most phenolic compounds belong to the flavonoids. Lignin, the primary substance of wood, is the most common member of this group. The following table outlines the most important groups of plant phenolic compounds.
|
The Most Important Classes of Phenolic Compounds in Plants |
||
|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
coumarin, isocoumarin |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(C6)n (C6 - C3 - C6)n |
catecholmelanine (condensed tannins) |
|
|
||
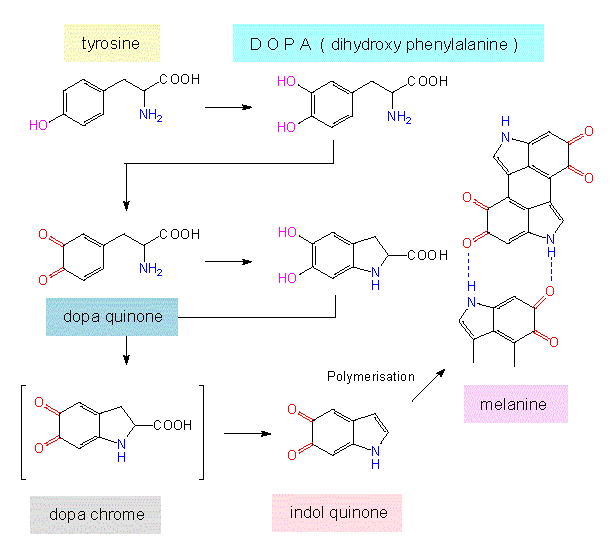
The starting product of the biosynthesis of most phenolic compounds is shikimate. Phenols are acidic due to the dissociability of their -OH group. They are rather reactive compounds and as long as no steric inhibition due to additional side chains occurs, they form hydrogen bonds. Consequently, many flavonoids have intramolecular bonds. Another important feature is their ability to form chelate complexes with metals. Also, they are easily oxidized and, if so, form polymers (dark aggregates). The darkening of cut or dying plant parts is caused by this reaction. They have usually an inhibiting effect on plant growth. Among the phenylpropanol derivatives of lower molecular weight are a number of scents like the coumarins, cinnamic acid, sinapinic acid, the coniferyl alcohols and others. These substances and their derivatives are at the same time intermediates of the biosynthesis of lignin.
Flavonoids: In 1975, the number
of identified flavonoids was estimated to be larger than 2000. Some
important representatives and their biological significance are
listed in the table below.
|
The Most Important Classes of Flavonoids and their Biological Significance |
||
|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
like those of tannins |
|
|
|
|
|
|
|
|
|
|
||
The basic structure of flavonoids is derived from the C15 body of flavone. They differ from other phenolic substances in the degree of oxidation of their central pyran ring. And, very fundamentally, also in their biological properties. While some classes (the flavonones, for example) are colourless, the members of other classes (the anthocyanes, for example) are always coloured and known as pigments of flowers or other plant parts. Anthocyanes are normally red or yellow, their colour is pH-dependent. Blue pigments are achieved by chelate formation with certain metal ions (FeIII or AlIII, for example).
The variability of the flavonoids is largely based on the hydroxylation and/ or methylation pattern of the three ring systems. A correlation between two flavonoids points often to a relationship between the producing plant species. They have therefore proven to be suitable traits for the study of the phylogenetic relations between higher plants. The quinones are another group of phenolic compounds. We have already met some of its members that function as co-factors. Accordingly, they do not actually belong to the secondary plant products but have to be counted among those of the basic metabolism. As has been mentioned before, phenolic compounds occur usually not unbound within plant tissues. They are mostly coupled to other molecules, often to glucosyl residues, but to sulphate- or acetyl-residues, too. One of the reasons may be that they are toxic when in a free state and are detoxified, at least partially, if coupled. Many low molecular weight compounds, for example thymol, are used in medicine as antiseptics due to their toxicity. Different types of bonds between flavonoids (for example anthocyanes) and a glycosyl residue lead to different derivatives that increase the range of flower colours (and colour shades). The glycosylation of flavonoids has an additional, ecologically not less important function. It has been brought into connection with pest protection and protection against other animals. Based on their biological functions, phenolic compounds can be classified as follows:
|
The Ecological Meaning of Some Phenolic Compounds for Plants |
||
|---|---|---|
|
|
|
where the effect was studied |
|
|
chalcons aurones yellow flavonoids flavones |
coreopsin in Coreopsis tinstoria aureusin in Anthirrhinum majus gossypetine-7-glucoside in Gossypium apigenin-7-glucoside in Bellis perennis |
|
|
isoflavones chalcons |
osajin in Maclura pomifera ocanin in Kyllingi brevifolia |
|
|
phenols phenolcarboxylic acids hydrocinnamic acid |
hydroquinone in Arctostaphylos sialic acid in Quercus falcata ferulic acid in Adenostoma |
|
|
tannines flavonols |
gallotannine in Quercus robur quercitine-glycosids in Gossypium |
|
|
phenolcarboxylic acids dihydrochalones |
protocatechunic acid in Allium phloridcine in Malus pumila |
|
|
phenylanthrenes isoflavanes pterocarpanes phenylpropanoids fucocoumarins |
orchinol in Orchis militaris vestitiol in Lotus corniculatus pisatin in Pisum sativum coniferyl alcohol in Linum usitiltissimum psoralen in Petroselinum crispum |
|
|
||