Botany online 1996-2004. No further update, only historical document of botanical science!
Glycolysis and the Citric Acid
Cycle
Glycolysis represents an
anabolic pathway common in both aerobic and anaerobic organisms.
Other sugars and polysaccharides have to be transformed into glucose
or one of its phosphorylated derivatives before being processed any
further. In the course of degradation, ATP is produced. Pyruvate may
be regarded as the preliminary final product of the degradation - in
a strictly formal sense - because it is here that the pathway
ramifies: pyruvate is hydrated under anaerobic conditions resulting
in either lactate (in lactic-acid bacteria) or ethanol (in yeast). If
glycolysis results in these final products, it is spoken of
fermentation. Under aerobic
conditions, pyruvate is fed into the citric acid cycle via an
intermediate product. This pathway produces a neat amount of energy
in the form of ATP. The starting product glucose is completely
oxydized to water and carbon dioxide.
In 1905, A. HARDEN and W. YOUNG noticed, that the degradation of
glucose stops if inorganic phosphate is not present in sufficient
amounts. It is necessary to phosphorylate sugar. They were able to
isolate a hexose diphosphate later on identified as
fructose-1,6-diphosphate and could prove that is was an intermediate
product of glucose degradation.
Glucose degradation was completely resolved in the years before
1940. The biochemists G. EMBDEN , O. MEYERHOFF, C. NEUBER, J.
PARNASS, O. WARBURG and G. and C. CORI contributed most.
Glycolysis, sometimes also called the
EMBDEN-MEYERHOFF-PARNASS-scheme consists of several reactions:
The pathway starts with an irreversible step, the ATP-consuming
phosphorylation of glucose. In the second, reversible reaction
glucose-6-phosphate is isomerized to fructose-6-phosphate.
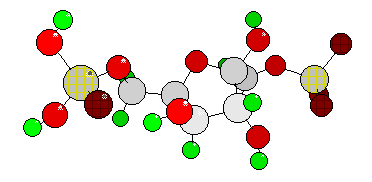 Consumption
of a second ATP leads to fructose-1,6-diphosphate. This step, too, is
irreversible as the delta G° of the phosphorylated sugar
is not sufficient to transfer the phosphate residue back to the ADP.
These reactions are the only irreversible steps of all glycolysis.
When drawing a linear structure of fructose-1,6-diphosphate ( =
fructose-1,6-bisphosphate or FDP, it becomes clear that both
phosphate groups are terminal so that the C-C linkage inbetween is
weakened (dipoles at both ends of the molecule). This leads to an
increased tendency of the molecule to disintegrate. With the aid of
the enzyme aldolase, the fragments D-glyceraldehyde-3-phosphate (GAP)
and dihydroxyacetone phosphate (DHAP) are thus produced. They can be
interconversed since they are aldose-ketose isomers. Under
physiological conditions far more dihydroxyacetone phosphate is
present. But it is glyceraldehyde-3-phosphate that is continuously
needed for subsequent reactions. It is steadily replaced by
interconversion of dihydroxyacetone phosphates.
Consumption
of a second ATP leads to fructose-1,6-diphosphate. This step, too, is
irreversible as the delta G° of the phosphorylated sugar
is not sufficient to transfer the phosphate residue back to the ADP.
These reactions are the only irreversible steps of all glycolysis.
When drawing a linear structure of fructose-1,6-diphosphate ( =
fructose-1,6-bisphosphate or FDP, it becomes clear that both
phosphate groups are terminal so that the C-C linkage inbetween is
weakened (dipoles at both ends of the molecule). This leads to an
increased tendency of the molecule to disintegrate. With the aid of
the enzyme aldolase, the fragments D-glyceraldehyde-3-phosphate (GAP)
and dihydroxyacetone phosphate (DHAP) are thus produced. They can be
interconversed since they are aldose-ketose isomers. Under
physiological conditions far more dihydroxyacetone phosphate is
present. But it is glyceraldehyde-3-phosphate that is continuously
needed for subsequent reactions. It is steadily replaced by
interconversion of dihydroxyacetone phosphates.
At this stage of glycolysis, the first oxidation ocurs though no
oxygen is present. As a consequene of the dehydration of ,
NAD+ is reduced. Glyceraldhyde-3-phosphate is at the same
time phosphorylated by addition of Pi.
The aldehyde group of glyceraldehyde-3-phosphate is thus
transformed into a carboxy-group that is esterized to a
phosphate. It is the exergonic aldehyde oxidation that drives
the synthesis of the acyl phosphate 1,3-bisphosphoglycerate
(1,3-BPG, also called 1,3-diphosphoglycerate). This molecule is
very unstable and looses one of its phosphate groups easily to
ADP. It is the first ATP-generating
step of glycolysis. Two molecules of ATP had been
invested and two are generated already during the first
energy-producing step.
Why two? The original C6 molecule has been
transformed into two C3 molecules so that we have to
double both gains and losses from now on.
The two following steps are not that interesting but the
dephosphorylation of phosphoenolpyruvate (PEP), the final
common step of glycolysis is important again. The free energy
of PEP hydrolysis is coupled to the synthesis of ATP to form
pyruvate. The subsequent fate of pyruvate is, as has been
mentioned above, dependend on the presence or absence of
oxygen. Anaerobic processing, also called fermentation, leads
to lactate or ethanol. Lactate is, for example, generated with
the help of the enzyme lactate dehydrogenase and consumes NADH
+ H+. Yeast cells growing under anaerobic conditions
produce ethanol with the aid of the enzymes pyruvate
decarboxylase and alcohol dehydrogenase. They, too, need NADH +
H+. The net gain of the fermentation process is
rather low since no more ATP is produced. Aerobic conditions,
in contrast, lead to the generation of acetyl-CoA:
pyruvate + NAD+ + CoA > acetyl-CoA +
CO2 + NADH + H+
The process is an oxidative decarboxylation since carbon
dioxide is set free while pyruvate is dehydrated (oxydized).
Thiamine pyrophosphate (TTP), the coenzyme of pyruvate
dehydrogenase plays a decisive part in this
reaction.
Coenzyme A is a cofactor (a prosthetic group) like
NAD+ and the others already discussed. The
terminal SH-group is the active centre that binds to a
C2 unit (an acyl residue) and transfers it to
another molecular group. Acetyl-CoA (activated acetic acid)
is on one hand the starting compound of the fatty acid
synthesis and on the other hand can the activated acetic
acid be coupled to oxaloacetate. This coupling is the first
reaction of the citric acid cycle.
Via a number of intermediate products,
oxaloacetate is finally regenerated, the reason why this
pathway is termed cycle.
But much more happens in its course:
In two steps of the pathway, carbon dioxide, one
of the two oxidation products of glucose is set free.
The C2 molecule fed into the cycle is thus
fully oxydized.
In four reactions, 2 H+ + 2
e- are generated, in three of them are they
transferred to NAD+, in one (in the
dehydration of succinate to fumarate) to FAD.
- Some of the intermediates are starting points of
biosynthetic pathways.
One molecule of ATP (GTP in animals) is produced.
Its generation is preceded by that of succinyl-CoA.
This compound contains a CS-CoA-linkage (a thioester
bond, delta G°: -8 kcal/mol = ca -33.6
kJ/mol). The breaking of this bond provides enough
energy for the generation of one molecule of ATP
(plants and bacteria) or GTP (mammals).
© Peter v. Sengbusch - Impressum
 Consumption
of a second ATP leads to fructose-1,6-diphosphate. This step, too, is
irreversible as the delta G° of the phosphorylated sugar
is not sufficient to transfer the phosphate residue back to the ADP.
These reactions are the only irreversible steps of all glycolysis.
When drawing a linear structure of fructose-1,6-diphosphate ( =
fructose-1,6-bisphosphate or FDP, it becomes clear that both
phosphate groups are terminal so that the C-C linkage inbetween is
weakened (dipoles at both ends of the molecule). This leads to an
increased tendency of the molecule to disintegrate. With the aid of
the enzyme aldolase, the fragments D-glyceraldehyde-3-phosphate (GAP)
and dihydroxyacetone phosphate (DHAP) are thus produced. They can be
interconversed since they are aldose-ketose isomers. Under
physiological conditions far more dihydroxyacetone phosphate is
present. But it is glyceraldehyde-3-phosphate that is continuously
needed for subsequent reactions. It is steadily replaced by
interconversion of dihydroxyacetone phosphates.
Consumption
of a second ATP leads to fructose-1,6-diphosphate. This step, too, is
irreversible as the delta G° of the phosphorylated sugar
is not sufficient to transfer the phosphate residue back to the ADP.
These reactions are the only irreversible steps of all glycolysis.
When drawing a linear structure of fructose-1,6-diphosphate ( =
fructose-1,6-bisphosphate or FDP, it becomes clear that both
phosphate groups are terminal so that the C-C linkage inbetween is
weakened (dipoles at both ends of the molecule). This leads to an
increased tendency of the molecule to disintegrate. With the aid of
the enzyme aldolase, the fragments D-glyceraldehyde-3-phosphate (GAP)
and dihydroxyacetone phosphate (DHAP) are thus produced. They can be
interconversed since they are aldose-ketose isomers. Under
physiological conditions far more dihydroxyacetone phosphate is
present. But it is glyceraldehyde-3-phosphate that is continuously
needed for subsequent reactions. It is steadily replaced by
interconversion of dihydroxyacetone phosphates.