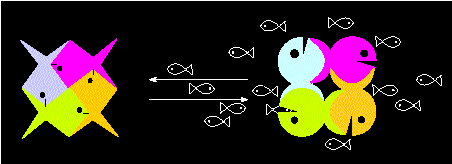Botany online 1996-2004. No further update, only historical document of botanical science!
Regulation of Enzyme Activities -
Allosteric Enzymes
The enzyme kinetics reviewed in the two sections above are valid
for enzymes of simple structures, i.e. for such enzymes that consist
of just one polypeptide chain which again has only one
substrate-binding site. In this context, the participation of
coenzymes can be neglected. The inhibition kinetics indicate that the
molecular conformation of an enzyme is not rigid but has a limited
flexibility. Such a reversible deformation occurs as soon as a
molecule has bound to the enzyme. It does not matter where it binds.
Molecules that induce structural changes of the enzyme are
collectively called effectors.
Among them may also be substrates.
Many enzymes that hold central switch positions in branched
biosynthesis pathways can temporarily be shut down by the final
product of one or both pathways. This is termed
end product inhibition. It
assures that the synthesis of a product is stopped as soon as enough
has been generated.
|
|
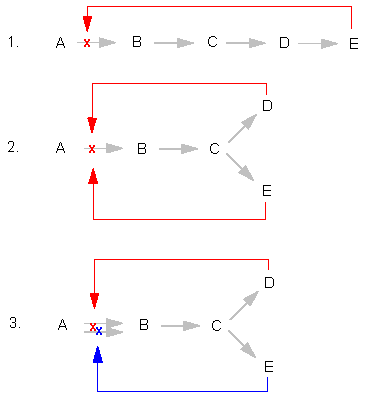
Regulation of the metabolism, feed-back inhibition by the
final product.
1. Simple feed-back inhibition. The final product (E)
inhibits the step from A to B.
2. Co-operative feed-back inhibition. Both final products
(D, E) inhibit the first step of their own synthesis
together.
3. Multivalent feed-back inhibition.
|
|

|
4. Inhibition at a
ramification of a biosynthesis pathway (sequential
inhibition)
|
A rather large group of enzymes consists of several polypeptide
chains. The complex (quaternary structure) may either be formed by
identical or different chains. In such a complex enzyme, the
transmission of information functions far better than between free
polypeptide chains.
The binding of a substrate molecule to, let us assume, one of four
polypeptide chains distorts at first this chain's structure. But the
distortion is immediately transmitted to the other three chains so
that their substrate binding sites are also changed.
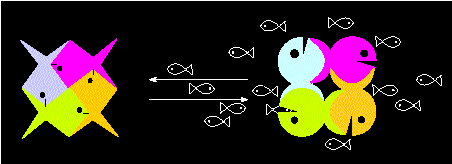
"Allosteric model" of a tetrameric protein. The transition
between one state of activity to another (transition between two
alternative conformations) is elicited by the substrate (the
little fishes) and occurs in a co-operative manner (according to
M. EIGEN and R. WINKLER, 1975)
Two possibilities exist: either a higher reactivity is achieved
(positive co-operation) or a
reduced one (negative
co-operation, desensibilization). Which alternative
applies for a special case can be recognized by its turn-over
kinetics. Enzymes of several polypeptide chains that are controlled
in this way are called allosteric enzymes.
A positive co-operation generates a sigmoid curve expressing that
the fist substrate molecule has been bound with a weak affinity while
the following ones are bound by the other chains that are by now in a
state of increased reactivity. The kM value of
allosteric enzymes is consequently no constant, but a function of the
substrate concentration.
A negative co-operation generates a signal that reduces the
substrate affinity.

Enzyme kinetics: Yellow
curve: Negative co-operation, red curve:
nNon-co-operative, lilac curve: Positive co-operation.
Enzymes with co-operative effects are always built from several
subunits (allosteric proteins).
The ability of enzymes to be controlled is of no lesser importance in
metabolism than their catalytic activity. It decides which of two
alternative pathways is chosen in a certain situation.
Since these control processes are reversible, it is guaranteed
that a new decision can be made at each moment so that the cell can
react without loss of time to changes in the substrate or product
concentration. Viewed from a point of scarce resources and permanent
energy crisis, it does become clear that these are mechanisms working
very efficiently.
The fast working, reversible property of enzymes to be regulated
is based on the instability of weak
interactions, since everything said about bonds and the
transmission of information elicited by them is caused by their
generation and breaking.
Beside this, enzymes may have the property to be regulated by
changes of their geometrical structure brought about by the
generation or breaking of covalent bonds. Such modifications are
without exception irreversible. Among them are the phosphorylation
and acetylation of enzymes as well as the breaking of a polypeptide
chain that renders an inactive enzyme active. A regulation can also
be achieved by the number of enzyme molecules per cell.
© Peter v. Sengbusch - Impressum