Botany online 1996-2004. No further update, only historical document of botanical science!
Redox reactions are among a cell's most important enzyme-catalyzed reactions. Oxidation and reduction refer to the transfer of one or more electrons from a donor to an acceptor, generally of another chemical species. The donor is oxidized, the acceptor reduced.
A substance that takes up electrons is called a reductant or reducing agent, while the electron donor is termed oxidant or oxidizing agent. Both together represent a redox couple:
electron donor < > e- + electron acceptor.
Oxidation-reduction reactions are accompanied by a change in free energy. The free energy is a measure for the tendency to donate or to accept electrons. The flow of electrons can be measured and is called redox potential or electromotive force.
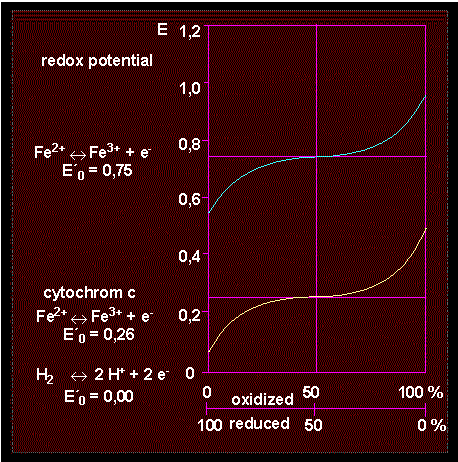
An element is found to be in its highest degree of oxidation when being in the compound that is poorest in energy. To depict redox reactions consistently, a common standard is needed, whose potential has arbitrarily been defined as zero. All other potentials refer to this standard. Standard reduction potentials are defined by convention with respect to the standard hydrogen half-reaction:
½ H2 < > H+ + e-
in which H+ is in equilibrium with H2(g) at pH 0, 25°C and 1 atm. The redox potential of any redox pair can now be measured and related to the standard reduction potential. The dimension of the potential is V.
It is described by the Nernst equation:
E = E0 - (RT / nF) ln [reduced substance] / [oxidized substance]
where
R is the gas constant,
T the absolute temperature,
F the faraday constant (23 kcal/mol or 96.6 kJ/mol),
n the number of electrons transferred during the reaction,
E the observed potential difference given in Volt and
E0 the standardized redox potential (referring to the hydrogen electrode).
Two conventions for the determination of the redox potential's sign exist:
Biochemical reactions are usually not measured at pH 0, but at the more physiological pH of 7 instead. The values obtained under these altered conditions are marked by the symbol E'0. E'0 values can be used to calculate delta G0 since the free energy is directly coupled to the redox potential:
delta G0 = - nFE'0
where n is the number of transferred electrons and F is the Faraday constant (23 kcal/mol or 96.6 kJ/mol).