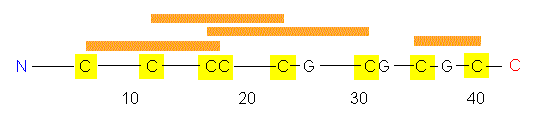Botany online 1996-2004. No further update, only historical document of botanical science!
Lectins
Lectins are sugar-binding proteins that agglutinate cells and/or
precipitate glycoconjugates (= molecules with a carbohydrate portion
like polysaccharides, glycoproteins, glycolipids and others).
Since they were originally only isolated from plant extracts and
were used for the agglutination of blood cells (erythrocytes), they
were at first called phytohaemagglutines. Later, it was found that
they can also be obtained from animal organs, especially those of
invertebrates and that they do not all bind to erythrocytes. W.C.BOYD
and E. SLAPEIGH did therefore coin the term lectin in 1954 (from lat.
legere = to choose). Lectins have at least two sugar binding
sites otherwise, their agglutination/precipitation behaviour cannot
be explained. Actually most of them are composed from two, four or
more usually identical subunits. Their specificity is defined by the
mono-or oligosaccharide that inhibits the agglutination
competitively. A choice of the best-known lectins is presented in a
table.
The affinity a lectin displays for cells or macromolecules
(ligands) is several orders higher than that for single sugars. This
led to the assumption that it were not only the carbohydrate parts
that were important for the binding but that certain, non-specific
protein-protein interactions (weak interactions) were stabilizing the
complex. A number of lectins, for example RCA, PNA, SBA, are known to
have an affinity to beta-D-galactosyl residues, but they show
a greatly varying binding ability for certain cells or glycoproteins.
The steric organization of the carbohydrates at the cell's or
molecule's surface have a great influence, they have to be accessible
for the lectin. The lectin-binding molecules are usually referred to
as lectin receptors due to the structural complexity of the ligands.
Although certain insecurities concerning their chemical
characterization exist, lectins are increasingly used in medicinal
pure research. They are used for the characterization of certain cell
types or fragments (like different membranes), to detect cells in
different states of development, to distinguish normal from tumour
cells, to mark the different states of the cell cycle and to separate
different cell types by affinity chromatography.
By linkage to fluorescence dyes (fluorescent markers), suitable
tags for the localization of
glycoconjugates in cells or at cell surfaces were gained. A
corresponding tagging with electron-dense substances like ferredoxin
or colloidal gold produced good helps for electron microscopy. Though
until now not many of such studies have been performed with plant
cells.
Although some of the mentioned properties are also shared by
antibodies, they do not have very much in common with lectins:
|

|
Antibody synthesis is inducible, lectin synthesis
not.
|
|

|
Antibodies can be directed against every determinant,
lectins only against a defined set of sugar molecules.
|
|

|
Antibodies are all build according to the same scheme
(they are related). Lectins belong to different protein
families. They are better compared to enzymes without
catalytic properties.
|
|

|
The protein structures of ConA and WGA are known. The
polypeptide chain of ConA is composed of 237 Amino acid
residues; its tertiary structure has an exceptionally high
proportion of beta - pleated
sheets, the active molecule is a tetramer.
|
Favin that occurs in Vicia faber, shows some surprising
correspondences with ConA. It has two polypeptide chains of unequal
length, alpha and beta. The alpha-chain is
homologous to section 70-119 of ConA, the beta-chain to
sections 120-237 and 1-69. These agreements are caused by a so-called
'cyclic permutation' of polypeptide sections. But as long as no
further data is available, it cannot be decided whether the
ConA-sequence is older and the flavin developed by taking out of the
middle section and an exchange of the two terminal fragments or
whether ConA was generated by fusion of the two polypeptide chains of
flavin.
The polypeptide chain of WGA has 164 amino acid residues, among
them numerous cysteine residues that are able to form disulfide
bonds.
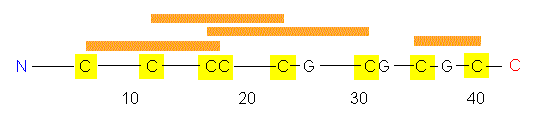
The amino acid chain folds into a tertiary
structure of four domains: A, B, C and D. As in many enzymes,
there is a cleft between A and B and between C and D. But this is not
the site of sugar-binding, that takes place at the outside of the
molecule. Could this be the reason why WGA does not act as an enzyme?
There are no 'special conditions' at the molecules' outside that are
characteristic for the active centre of an enzyme.
The comparison of WGA's and ConA's tertiary structures shows that
no similarities are shared. But the domains A, B, C and D of WGA are
all very similar. It may thus be assumed that the gene for WGA
developed by two consecutive duplications of the original gene.
Serological cross-reactions showed that different gramineae
contain lectins that are related serologically and most likely also
structurally with WGA. This is obviously a protein family. The
lectins of leguminoses are all metalloproteins. Whether this also
points to a common phylogenetic family remains unclear.
What importance do lectins have for the plant? It is well-known
that sugar-residues can be found at cell surfaces and that single
cell types and their different developmental stages differ in their
carbohydrate pattern.
It was therefore not far-fetched to find out whether lectins take
part in recognition processes. The role of certain lectins was
proven:
|

|
cell-cell-recognition, for example mating types of algae
(Chlamydomonas and others)
|
|

|
pollen-stigma
interactions.
|
|

|
recognition of symbiotic partners, for example in the
recognition process of Rhizobium-species and their
specific host plants (Leguminoses).
|
|

|
recognition of parasitic fungi and subsequent induction
of the plant's defence
mechanisms.
|
Many lectins are localized within the cell where they may be
present in considerable amounts but their role is largely unknown.
They react with storage proteins and their affinity to the species
own storage proteins is far greater than that to foreign proteins.
This led to the assumption (S.-K. BASHA and R. M. ROBERTS, 1981) that
they complex proteins and thus transform them into a compact,
insoluble state that makes them easier to store than a soluble state
would.
Many lectins are toxic and may therefore protect the plant from
being eaten away. The lectin of pea (Phaseolus vulgaris), for
example, is deadly for the bug Calosobruchus maculatus. The
agglutinin of Ricinus communis (RCA) contains a component that
is highly poisonous for animals and man: ricin. Animal experiments
showed that some lectins support cell division at low concentrations
(mitogenes). If this is of importance for the plant remains
unclear.
© Peter v. Sengbusch - Impressum
![]()
![]()
![]()
![]()