Botany online 1996-2004. No further update, only historical document of botanical science!
The Calpha-atom of amino acids carries four different groups: an amino group, a carboxyl-group, an H-atom and a side chain. The twenty amino acids occurring in all living things are marked by a three or a one letter code.
The Calpha-atom of amino acids carries both a carboxyl-group and an amino-group besides an H-atom and a side chain R.

The formula shows that they are optically active compounds. Most naturally occurring amino acids and all that can be found in proteins belong to the L-group. Both the carboxyl- and the amino-group can be ionized (amphoteric ions), the other carbon atoms, too, may be chiralic (optically acive!). Lysine, chosen as an example here, shows the different ways of drawing the molecule:
|
|
|
|
|
|
2,6-diamino capronic acid |
lysine in Fischer projection: |
shortened chemical |
space-filling model |
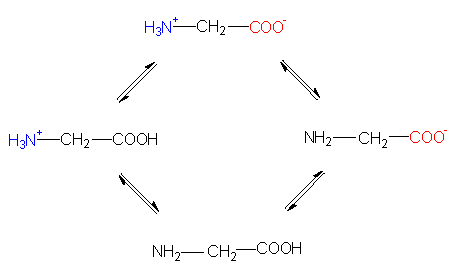
State of ionization in an acidic, a neutral and an alkaline solution.
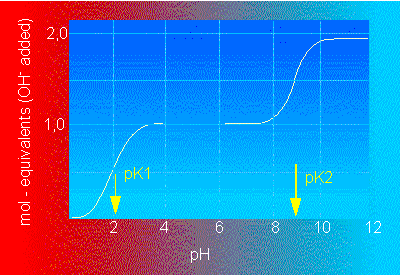
Titration curve of glycine (with two pK-values).
The single amino acids differ in the side chain R. Twenty different
amino acids can be found in proteins. Some more occur as free forms
especially in plants and several of them will be introduced
later.
The twenty amino acids are characterized by different charges, sizes, abilities to form hydrogen bonds and by different chemical reactivities. A part of them has short, linear or branched non-polar aliphatic chains as side chains. Three amino acids have aromatic or heterocyclic side chains. Two amino acids contain a hydroxyl-group, two further sulphur. Again two others carry additional carboxyl- or amino-groups and one has an imidazol group. These groups can be ionized. Two last amino acids have amidized carboxyl-groups so that the ability to become ionized is lost.
It is common in biochemistry to mark amino acids with a
three letter code, for example
gly for glycine. Since computers became usual aids for the evaluation
of protein-biochemical data, it has mostly been replaced by a
one letter code.
Hydrophobic (water-repellent) non-polar amino acids
Polar, but uncharged amino acids:
Polar, charged amino acids:
Acidic amino acids
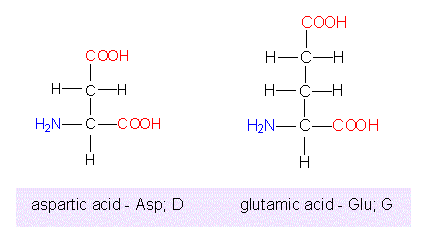
aspartic acid, glutamic acid
Basic amino acids
|
|
Ala |
Arg |
Asn |
Asp |
Cys |
Glu |
Gln |
Gly |
His |
Ile |
Leu |
Lys |
Met |
Phe |
Pro |
Ser |
Thr |
Trp |
Tyr |
Val |
|
acidic |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
acyclic |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
aliphatic |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
aromatic |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
basic |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
buried |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
charged |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
cyclic |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
hydrophobic |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
large |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
medium |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
negative |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
neutral |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
polar |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
positive |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
small |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
surface |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|