C. References
XI. The Chl b Biosynthetic Pathway
Fig. 4: Synopsis of all Possible Reactions that May Result in the Formation of MV Chl b.
The reactions between ALA and DV Proto are shown in more detail in Fig. 1. The various Chl a carboxylic biosynthetic routes are discussed in sections I to VIII. DV= divinyl; MV = monovinyl; Mg-Proto = Mg-protoporphyrin IX; Mpe = Mg-Proto monomethyl ester.
The demonstration of metabolic pathways is a multistep process. It involves at least three stages: (a) the detection and characterization of metabolic intermediates, (b) the demonstration of precursor-product relationships between putative intermediates, and (c) purification and characterization of enzymes involved in the metabolic interconversions. These criteria will be applied in our evaluation of the experimental evidence that supports the operation of a multibranched Chl b biosynthetic pathway in green(ing) plants.
A. Determination of Precursor-Product Relationships In Vivo
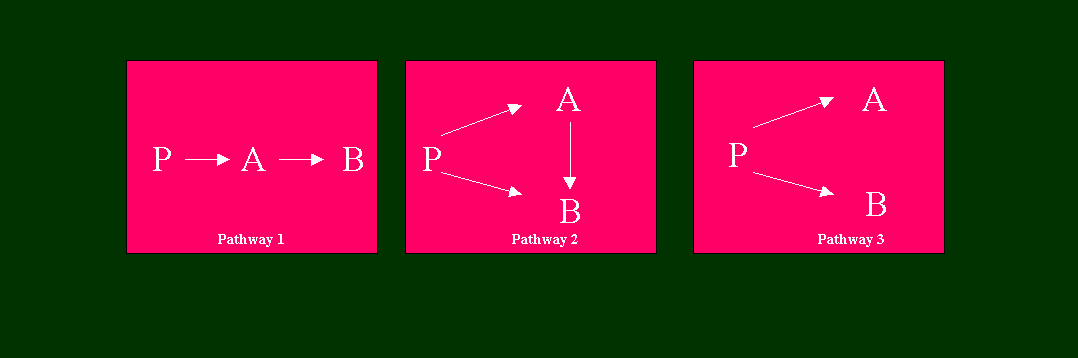
Fig. 5. The Three Possible Irreversible Precursor-Product Relationships Between Two Precursors (P, A) and One End Product (B) (Adapted from Rebeiz et al, 1988)
In discussing the Chl b biosynthetic pathway, use will be made of kinetic analysis of precursor-product relationships in vivo. In 1988, equations were derived to investigate possible precursor-product relationships in branched, and interconnected pathways (Rebeiz et al, 1988, Tripathy and Rebeiz, 1988). It was shown that for any two compounds A and B, formed from a common precursor P such as ALA, and having a possible direct precursor-product relationship between them, for any number of time intervals t1 to t2, the following equation describes the relationship between the specific radioactivity of compound A, possible radiolabel incorporation from compound A into compound B, and the net synthesis of compound B from compound A (Rebeiz et al, 1988):
QB2 = (*A1 + *A2)/2).(*B2) (Eq. 1) where:
QB2 = amount of radiolabel incorporated into compound B during time interval t1 - t2;
*A1,*A2 = specific radioactivity of compound A at the beginning and end of time interval t1 - t2 respectively.
*B2 = amount of compound B synthesized during time interval t1 - t2.
%Conversion = 100 - [(|Exp-QBX|/Exp)100] Eq. 2.
where:
% Conversion = maximum possible percent conversion of compound A to compound B during any time interval X.
Exp = actual 14C-incorporation into compound B by the end of time interval X, as determined experimentally.
QBX = theoretical 14C-incorporation into compound B by the end of time interval X, as calculated from Eq. 1.
|Exp-QBX| = absolute difference between the experimental and theoretical 14C-incorporation of precursor P into compound B during time interval X.
B.