F. References
VIII. The Chl a Carboxylic Biosynthetic Routes: Reactions Between chlorophyllide (Chlide) a and Chlorophyll (Chl) a
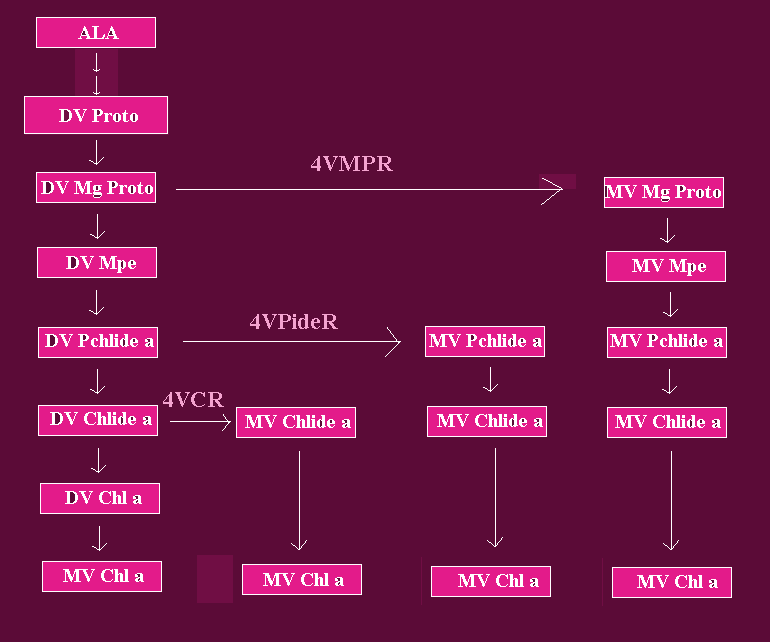
Fig. 2: Reactions of the DV and MV Carboxylic Chl a Biosynthetic Routes Between Mg-Proto and Chl a
The reactions between ALA and DV Proto are shown in more details in Fig. 1. DV= divinyl; MV = monovinyl; Mg-Proto = IX; Mpe = Mg-Proto monomethyl ester; Pchlide a= protochlorophyllide a; Chlide a = Chlorophyllide a; Chl a = chlorophyll a; 4VMPR = [4-vinyl] Mg-Protoporphyrin IX reductase; 4VPR = [4-vinyl] protochlorophyllide a reductase; 4VCR = [4-vinyl] chlorophyllide a reductase. Arrows joining the DV and MV branches refer to reactions catalyzed by [4-vinyl] reductases. Triple-lined arrows point to putative reactions. Adapted from Rebeiz et al, 1994.
In higher plants, most of the Chl a is esterified with a long chain fatty alcohol, phytol (C20H39OH). Esterificatrion of Chl a appears to follow different routes in etiolated and green tissues.
A. Esterification of Chlide a in Etiolated Tissues
That in etiolated tissues esterification of Chlide a was a complex reaction involving more than one step evolved from the observation that treatment of etiolated wheat seedlings with herbicides resulted in the accumulation of geranylgeraniol (GG) and dihydrogeranylgeraniol (DHGG)-containing Chl (Rudiger et al, 1976). This was followed by the demonstration of Chlide a esterification with GG in a cell-free system from maize shoots (Rudiger et al, 1977). Further work dealing with the identification of various esterified Chlide a in tiolated tissues subjected to a brief light treatment followed by dark incubation, led to the proposal that Chlide a is first esterified with geranylgeraniol (GG) to yield Chl a GG, which is reduced stepwise to Chl a dihydroGG (DHGG), tetrahyddroGG (THGG) and finally to hexahydroGG, i. e. phytylated Chl a (Schoch, 1978).
The above hypothesis was confirmed in cell-free systems from various etiolated plant tissues. It was demonstrated that in irradiated etioplast-membrane fractions prepared form oat seedlings, [1-3H]GG and its monophosphate were incorporated into Chl a only in the presence of exogenous ATP, whereas incorporation of activated [1-3H]GG pyrophosphate (PP) did not require ATP (Rudiger et al, 1980). In order to distinguish this enzymatic activity from chlorophyllase it was named Chl synthetase. Conversion of ChlGG in vitro to Chl a phytol by hydrogenation required the addition of exogenous NADPH; NADH was not a cofactor (Benz et al, 1980). Enzymic hydrogenation of ChlGG to Chl a phytol was inhibited by anaerobiosis (Schoch et al, 1980). Substrate specificity investigations indicated that Chl synthetase requires a chlorin derivative that contains Mg as the central metal ion. A hydrogenated ring D was mandatory since Pchlide a with a double bond at position 7-8 of the macrocycle was not a substrate (Benz and Rudiger, 1981; Helfrich and Rudiger, 1992; Rudiger, 1993). In etiolated tissues however, direct esterification of endogenous Chlide a with exogenous phytol in the presence of added ATP , and Mg was also observed, which led to the proposal that the conversion of Chlide a to Chl a may follow different biosynthetic routes having different substrate and cofactor requirements, depending on the stage of plastid development (Daniell and Rebeiz, 1984). In oat etioplasts, the relative substrate specificities for GGPP, PhyPP and farnesylpyrophosphate amounted to 6, 3, and 1 respectively (Rudiger, 1993).
Chlorophyll synthetase is present mainly in the prothylakoid and prolameelar body of etioplasts (Rudiger, 1993). Prolamellar body disaggregation and Chlide a esterification appear to be closely related. It appears that Chlide a formed in the prolamellar body can migrate with Pchlide-oxidoreductase to the prothylkakoid membranes during light-dependent dissociation of prolamellar bodies (Rudiger, 1993)
B. Esterification of Chlide a in Greening and Green Tissues
Illumination of etiolated tissues with white light leads to a slow decrease in Chl synthetase activity (Rudiger, 1993). Nevertheless Chl synthetase activity is observed in mature chloroplasts (Soll and Schultz, 1981). In spinach chloroplasts, the relative substrate specificity for exogenous GGPP and PhyPP were 1 and 4 respectively for esterification of Chlide a (Sol et al, 1983).
In Arabidopsis thaliana a nuclear encoded gene, G4, was identified which exhibited homology to the product of the Rhodobacter capsulatus bchG locus which is involved in the esterification of bacteriochlorophyllide with GG (Gaubier et al, 1996). The relationship between gene G and bchG was confirmed by isolation and sequencing of a corresponding full length cDNA. The gene appears to consist of 14 exons, some of which were very short. Southern and Northern analyses showed that gene G is a single copy gene and its transcripts were only detected in green or greening tissues. This in turn raises the issue of Chl a biosynthetic heterogeneity at the terminal stages of Chl a biosynthesis, and whether conversion of Chlide a to Chl a is catalyzed by one or more enzymes in higher plants.
C. Conversion of DV Chlide a to DV Chl a
The major fate of DV Chlide a resides in its conversion to MV Chlide a (see above) and Chl a. However under certain cicumstances, DV Chlide a is converted to DV Chl a by esterification. For example in the ON 2 corn mutant (Bazzaz, 1981), the major fate of DV Chlide a is its conversion to DV Chl a (Rebeiz et al, 1983). So is the case in the prochlorophyte picoplankton of the subtropical waters of the North Atlantic as well as in the picoplankton of the euphotic zone of the world tropical and temperate oceans, and the Mediterranean sea, where DV Chl a and b are the predominant Chl species (Veldhuis and Kraay, 1990; Chisholm et al, 1990; Chisholm et al, 1992; Goerike and Repeta, 1992).
In etiolated tissues of dark DV plant species, it has been repeatedly observed that when the mixed MV-DV Pchlide a is photoreduced to a mixed MV-DV Chlide a pool by a 2.5 ms light pulse, some of the nascent DV Chlide a is rapidly converted to DV Chl a during the first 30 s of dark incubation (Rebeiz, et al, 1983). The whereabout of this DV Chl a is rapidly obscured however by the accumulation of 2-MV Chl a. The conversion of the nascent DV Chl a to MV Chl a by a putative [4-vinyl] Chl a reductase is a strong possibility. At this point it is not known whether the conversion of DV Chlide a to DV Chl a proceeds via phytol, GG or both.
D. Conversion of DV Chlide a to MV Chl a Via MV Chlide a
In etiolated plant species, which form DV Pchlide a in darkness and in the light (Ioannides et al, 1994), DV Pchlide a is photoconverted to DV Chlide a (Belanger and Rebeiz, 1980, Duggan and Rebeiz, 1982). DV Chlide a is rapidly converted into MV Chlide a by reduction of the vinyl group at position 4 to ethyl. The reaction is catalyzed by [4-vinyl]-Chlide a reductase (4VCR) (Parham and Rebeiz, 1992, 1995). The enzyme is bound to the plastid membranes and requires NADPH for activity. It exhibits maximum activity at 30 C and at a pH of 6.3. During greening, 4VCR activity drops in dark divinyl plant species such as cucumber and vanishes from dark monovinyl (DMV) species such as wheat corn and barley (Haggag et al, under review). On the basis of these results, it was concluded that conversion of DV Chlide a to MV Chl a via the afore-mentioned route takes place early during the greening process, and is disabled in green DMV-light-dark MV (DMV-LDMV) plant species (see greening group affiliation of plants) such as corn barley and wheat (Abdel-Mageed et al, 1997). It is not clear whether this specific conversion of MV Chlide a to MV Chl a proceeds via phytol, GG or both.
E. Conversion of MV Chlide a to MV Chl a
Conversion of MV Chlide a to MV Chl a can proceed via two distinct biosynthetic routes which are described below.
1. Conversion of DV Pchlide a to MV Chl a via MV Pchlide a and MV Chlide a
Etiolated, DMV plant species such as French bean and corn, which form and accumulate MV Pchlide a upon proplonged incubation in darkness, regenerate considerable amounts of DV Pchlide a following a brief light treatment of a few ms, upon returning the plants to darkness (Belanger et al, 1982). In the light, regenerated DV Pchlide a is partially converted to MV Pchlide a, a reaction catalyzed by [4-vinyl] Pchlide a reductase (4PideR) (Tripathy and Rebeiz, 1989). MV Pchlide a is then photoconverted to MV Chlide a and the latter to MV Chl a either via phytol, GG esterification or both.In green DDV-LDDV plant species such as cucumber in which DV Pchlide a biosynthesis and accumulation is very active in darkness and in daylight, 4VCR activity is much reduced (Abdel Mageed et al, 1997). In this case too, DV Pchlide a is converted to MV Pchlide a by 4PideR (Tripathy and Rebeiz, 1989), and the resulting MV Pchlide a is photoconverted to converted to MV Chlide a and the latter is esterified to MV Chl a with phytol, GG or both.
2. Conversion of MV Mpe to MV Chl a via MV Pchlide a and MV Chlide a
In green DMV-LDMV plant species such as barley wheat and corn, 4VCR is completely inactive (Abdel-Mageed et al, 1997). Also in such plants, photoconversion of MV Pchlide a to MV Chlide a is catalyzed by POR-B (Reinbothe et al, 1995a, 1995b). In these green plants, the major route of MV Chl a formation is most probably via conversion of MV Mg-Proto to MV Chlide a, and the latter to MV Chl a by esterification with phytol, GG, or both. In these species, MV Mg-Proto is formed by 4-vinyl reduction of DV Mg-Proto, a reaction catalyzed by [4-vinyl] Mg-Proto reductase (Kim and Rebeiz, 1996).References
- Rudiger, W., Benz, J., Lempert, U., Schoch, S. and Steffens, D. (1976). Inhibition of phytol accumulation with herbicides: Geranylgraniol and dihydrogranylgeraniol-containing chlorophyll from wheat seedlings. Z. Pflanzenphysiol. 80: 131-143.
- Rudiger, W., Hedden, P., Kost, H. P., and Chapman, D. J. (1977). Esterification of chlorophyllide by geranylgeranyl pyrophosphate in a cell-free system from maize shoots. Biochem. Biophys. Res. Commun. 74: 1268-1274.
- Schoch, S. (1978). The esterification of chlorphyllide a in greening bean leaves. Z. Naturforsch. 33 c: 712-714.
- Rudiger, W., Benz, J., and Guthoff, C. (1980). Detection and characterization of activity of chlorophyll synthetase in etioplast membranes. Eur. J. Biochem. 109: 193-200
- Benz, J., Wolf, C., and Rudiger, W. (1980). Chloropohyll biosynthesis: hydrogenation of geranylgeraniol. Plant Sci. Lett. 19: 225-230.
- Schoch, S., Hehlein, C., and Rudiger, W. (1980). Influence of anaerobiosis on chlorphyll biosynthesis in greening oat seddlings (Avena sativa L.). Plant Physiol. 66: 576-569.
- Benz, J., and Rudiger, W. (1981). Chlorphyll biosynthesis: Various chlorphyllides as exogenous substrates for chlorophyll synthetase. Z. Naturforsch. 36c: 51-57.
- Helfrich, M., and Rudiger, W. (1992). Various metallopheophorbides as substrates for chlorophyll synthetase. Z. Naturforsch. 47c: 231-238.
- Rudiger, W. (1993). Esterification of chlorphyllide and its implication for thylakoids development. In: Pigment-Protein Complexes in Plastids: Synthesis and assembly. Sundqvist, C., and Ryberg, M. (eds.). pp: 219-240. Academic Press, New York.
- Soll, J., and Schultz, G. (1981). Phytol synthesis from geranylgeraniol in spinach chloroplasts. Biochem. Biophys. Res. Commun. 99: 907-912.
- Soll, J., Schultz, G., Rudiger, W. and Benz, J. (1983). Hydrogenation of grenylgeraniol:Two pathways exist in spinach chloroplasts. Plant Physiol. 71: 849-854.
- Daniell, H. and Rebeiz, C. A. (1984). Bioengineering of photosynthetic membranes. Requirement of magnesium for the conversion of chlorophyllide a to chlorophyll a during the greening of etiochloroplasts in vitro. Biotech. Bioeng. 27: 481-487.
- Gaubier, P., Wu, H. J., Laudie, M. D. and Grellet, F. (1995). A chlorphyll synthetase gene from Arabidopsis thaliana. Mol. Gen. Gent. 249: 58-64.
- Bazzaz (1981). New chlorophyll chromophore isolated from a chlorophyll deficient mutant of maize. Photobiochem. Photobiophys. 2: 199-207.
- Rebeiz, C. A., S. M. Wu, M. Kuhadja, H. Daniell, and E. J. Perkins. Chlorophyll biosynthetic routes and chlorophyll a chemical heterogeneity in plants. Mol. Cell. Biochem. 58:97-125.
- Veldhuis, M. J. W., and G. W. Kraay, (1990) Vertical distribution of pigment composition of a picoplanktonic prochlorophyte in the subtropical north Atlantic: A combined study of pigments and flow cytometry. Mar. Ecol. Progr Ser. 68: 121-127.
- Chisholm, S. W., R. J. Olson, E. R. Zettler, R. Goericke, W. J. B., and N. A. Welschmeyer, (1990) A novel free-living prochlorophyte abundant in the oceanic euphotic zone. Nature. 334: 340-343.
- Chisholm, S. W., S. Frankel, R. Goerike, R. Olson, R. Palenic, B. Urbach, J. Waterbury, and E. Zettler, (1992) Prochlorococcus marinus nov.gen. sp.: an oxyphototropic marine prokaryote containing divinyl chlorophyll a and b. Arch. Mikrobiol. 157: 297-300.
- Goerike, R., and D. Repeta, (1992) The pigments of Prochlorococcus marinus. The presence of divinyl-chlorophyll a and b in a marine prochlorophyte. Limnol. Oceanogr. 37: 425-433.
- Ionannides, I. M., D. A. Fasoula, K. R. Robertson, and C. A. Rebeiz, (1994) An evolutionary study of chlorophyll biosynthetic heterogeneity in green plants. Biochem. Sys. Ecol. 22: 211-220..
- Belanger, Faith C. and C. A. Rebeiz (1980). Chloroplast Biogenesis 30. Chlorophyll(ide) (E459 F675). The first detectable products of divinyl and monovinyl protochlorophyll photoreduction. Plant Sci. Lett. 18: 343-350.
- Duggan, J. X. and C. A. Rebeiz (1982). Chloroplast Biogenesis 42. Conversion of divinyl chlorophyllide a to monovinyl chlorophyllide a in vivo and in vitro. Plant Sci. Lett. 27: 137-145
- Parham, R., and C. A. Rebeiz, (1992) Chloroplast Biogenesis: [4-Vinyl] chlorophyllide a reductase is a divinyl chlorophyllide a-specific NADPH-dependent enzyme. Biochemistry. 31: 8460-8464.
- Parham, R., and C. A. Rebeiz, (1995) Chloroplast Biogenesis 72: A [4-vinyl] chlorophyllide a reductase assay using divinyl chlorophyllide a as an exogenous substrate. Anal. Biochem. 231: 164-169.
- Abdel-Mageed, A., K. F. El Sahhar, K. R. Robertson, R. Parham, and C. A. Rebeiz (1997). Chloroplast Biogenesis76: Two novel monovinyl and divinyl light-dark greening groups of plants and their relationships to the chlorophyll a biosynthetic heterogeneity of green plants. Under Review.
- Belanger, F. C., J. X. Duggan, and C. A. Rebeiz (1982). Chloroplast Biogenesis. Identification of chlorophyllide a (E458 F674) as a divinyl chlorophyllide a. J. Biol. Chem. 257: 4849-4858.
- Tripathy, B. C. and C. A. Rebeiz (1989). Chloroplast Biogenesis 60: Conversion of divinyl protochlorophyllide to monovinyl protochlorophyllide in green(ing) barley, a dark monovinyl light divinyl plant species. Plant Physiol. 87: 89-94
- Reinbothe C, Apel, K. and Reinbothe S. (1995a). A light-induced protease from barley plastids degrades NADPH:protochlorophyllide oxidoreductase complexed with chlorophyllide. Mol. Cell. Biol. 15: 6206-6212.
- Reinbothe S., Reinbothe C., Holtorf H., and Apel, K. (1995b). Two NADPH:protochlorophyllide oxidoreductases in barley: evidence for the selective disappearance of POR A during the light-induced greening of etiolated seedlings. Plant Cell 7: 1933-1940.
- Kim, J. S., and Rebeiz, C. A. (1996). Chloroplast Biogenesis: Origin of the Chl a biosynthetic heterogeneity in higher plants. J. Biochem. Mol. Biol. 29 :327-334.
Go Back to Main Menu