Chlorophyll a Biosynthetic Pathway
Topics
Click on a topic to go directly to it.
VII. The Chl a Carboxylic Biosynthetic Routes: (Photo)Conversion of Protochlorophyllides (Pchlides) a to Chlorophyllide (Chlide) a
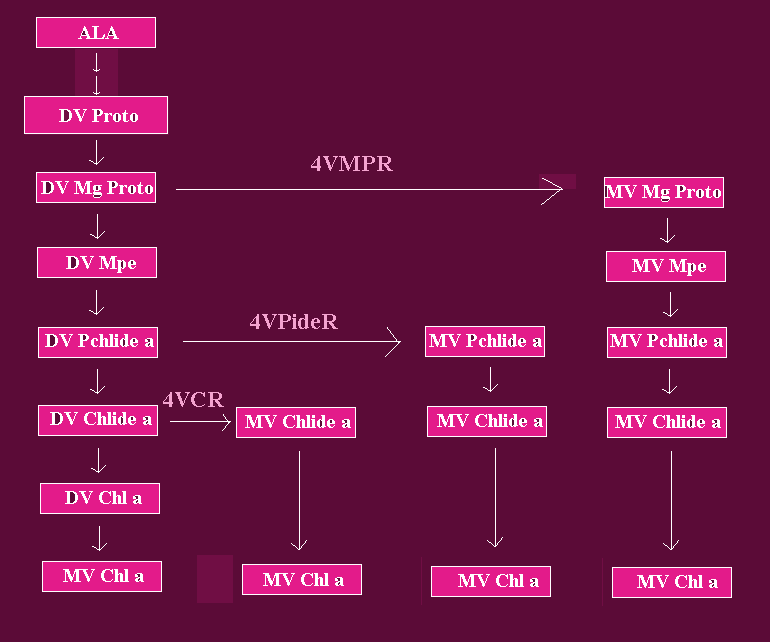
Fig. 2: Reactions of the DV and MV Carboxylic Chl a Biosynthetic Routes Between Mg-Proto and Chl a
The reactions between ALA and DV Proto are shown in more details in Fig. 1. DV= divinyl; MV = monovinyl; Mg-Proto = IX; Mpe = Mg-Proto monomethyl ester; Pchlide a= protochlorophyllide a; Chlide a = Chlorophyllide a; Chl a = chlorophyll a; 4VMPR = [4-vinyl] Mg-Protoporphyrin IX reductase; 4VPR = [4-vinyl] protochlorophyllide a reductase; 4VCR = [4-vinyl] chlorophyllide a reductase. Arrows joining the DV and MV branches refer to reactions catalyzed by [4-vinyl] reductases. Triple-lined arrows point to putative reactions. Adapted from Rebeiz et al, 1983, 1994.. The various routes are believed to lead to the formation of MV Chl a, at different sites of the photosynthetic membranes.
The notion that the Pchl(ide) a H apoprotein acts as a shuttling photoenzyme that catalyzes the conversion of Pchlide a to Chlide a was first proposed by Sironval et al (1967). In this work the authors report that Pchl(ide) a (E647 F 657), with a red excitation maximum at 647 nm and a red emission maximum at 657 nm, is photoconverted to Chlide a (E676 F690). The latter shifts in darkness to a Chlide a (E682 F697) species. At this stage, the authors suggest that the apoprotein discharges the newly formed Chlide a and picks up another Pchlide a which may be photoconverted to Chlide a via a similar cycle. The spectral shifts described by Sironval et al were confirmed by Gassman et al (1968), and Bonner (1969). The concept of shuttling photoenzyme was also compatible with the photoconversion of photoinactive Pchlide a 633 to phototransformable Pchlide a 650 reported by Gassman (1973).
Also discussed will be the light-independent conversion of Pchlide a to Chlide a. Although light-independent Pchlide a reduction has been known to occur in gymnosperms its occurrence in angiosperms is novel (Adamson and Packer, 1984).
A. Protochlorophyllide a (Photo)Oxidoreductases
Instead of one Pch(ide) a reductase, it is now realized that two Pchl(ide) a photooxidoreductases are functional in plants. That notion evolved slowly, over a certain number of years and is certainly compatible with the heterogeneity of the Chl a biosynthetic pathway (Rebeiz et al, 1983, 1994).
1. NADPH-Protochlorophyllide a (Photo)oxidoreductase A (POR A, or PCR)
A significant step in the understanding of Pchlide a photoreduction was achieved with the realization that NADPH is the hydrogen donor for the reaction (Griffiths, 1974). This was followed by the suggestion that the shuttling photoenzyme, NADPH and Pchlide a form a photoactive ternary Pchlide a NADPH-enzyme complex with a red absorption maximum at 652 nm (Griffiths, 1978). Equally important was the purification of POR A from etiolated barley (Apel et al, 1980). The enzyme consists of one polypeptide (Mr 36000) with two to three bound Pchlide a chromophores. It is synthesized in the cytoplasm as a precursor protein of about 44 kDa. The transit sequence of about 8 kDa, is hydrolyzed when the enzyme is transported into the plastid (Apel, 1981). The size of PorA reported by various authors depends on the plant species and varies from 33-38 kDa (see Review by Schulz and Senger, 1993). At least four different isozymes may be present in plants (Ikeuchi and Murakami, 1982; Dehesh et al, 1986)
It is has been demonstrated that during greening of etiolated tissues a rapid decline of POR A is observed. After six hours of continuous illumination, when the rate of Chl a accumulation is at its peak, only traces of the POR A protein are detected (Santel and Apel, 1981). This led Santel and Apel to propose that in etiolated tissues POR A functions only for a short period to time after the onset illumination. The disappearance of POR A from etiolated tissues during greening was confirmed by Kay and Griffiths (1983).
2. Protochlorophyllide a oxidoreductase B (POR B)
It has recently been demonstrated that in Arabidopsis thaliana and Barley, two different genes PorA and PorB ( with about 75 % homology) code for two different protochlorophyllide oxidoreductases, namely POR A and POR B (Armstrong et al, 1995; Holtorf et al, 1995). POR A is synthesized in the dark and constitutes the bulk of the crystalline prolamellar body of etioplasts. However the transcription of its gene is turned off in the light and the enzyme is rapidly degraded by a light-induced protease (Reinbothe et al, 1995a, 1995b). On the other hand, the PorB gene is transcribed in darkness and in the light, and the transcripts are translated continuously into the enzyme which is responsible for Chl a biosynthesis and accumulation in daylight.
It should be emphasized that etiolation and prolamellar body formation are not abnormal phenomena. They are part of the natural succession of the dark (night) and light (daylight) cycles during photoperiodic greening (Cohen and Rebeiz, 1978; Rebeiz, and Rebeiz, 1986). As such it is expected therefore, of POR A and POR B, to play definite roles during Chl a biosynthesis and accumulation under natural photoperiodic conditions. Furthermore it is well established that the activities of the DV and MV monocarboxylic Chl a biosynthetic routes differ among plant species, as well as during the dark and light phases of the photoperiod (Rebeiz, 1987; Rebeiz et al, 1994). What is presently unclear is the specific relationship of POR A and POR B activities to the DV and MV heterogeneity of the Chl a biosynthetic pathway (Fig. 2).
B. Light-Independent Protochlorophyllide a Reductase
Algae, ferns, mosses, and the cotyledons of most gymnosperms are capable of converting Pchlide a to Chlide a in the absence of light, via a light-independent Pchlide a reductase (Kirk and Tilney-Basset, 1967; Ryberg and Sundqvist, 1991; Shulz and Senger, 1993).
In Angiosperms, light is required for the formation of photosynthetic pigment-protein complexes and the accumulation of the massive amounts of Chl found in photosynthetic membranes. Adamson and coworkers pioneered the notion that a certain amount of Chl a biosynthesis can take place in darkness via the action of a light-independent Pchlide a reductase (Adamson and Packer, 1984). Incorporation of 14C-glutamate and 14C-ALA into barley leaves and barley etiochloroplasts in darkness has been confirmed by Tripathy and Rebeiz (1987). It should be emphasized that the amount of Chl a formed in darkness is very small, and its biological significance is unknown. Obviously light is required for the massive formation of the pigment-protein complexes that catalyze the process of photosynthesis in angiosperms.
C. Photoreduction Intermediates and Spectral Shifts During Photoreduction of Protochlorophyll(ide) a H (E550 F655)
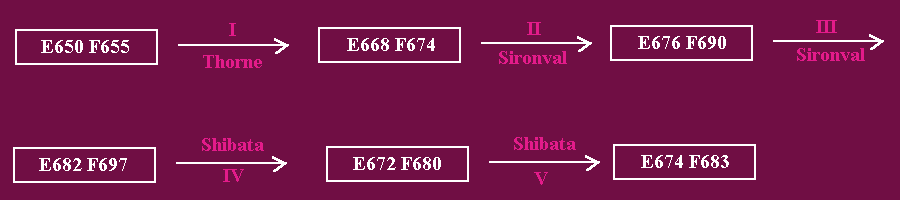
Fig. 3. Spectral Shifts of the Ternary Pchlide a-NADPH-Apoprotein Complex During the Photoconversion of Pchlide a to Chl(ide) a. "E" and "F" refer to the red fluorescence excitation and emission maxima respectively at 77 K.
The photoconversion of LW t-Pchl(ide) a (E650 F655) (the ternary Pchlide a-NADPH-apoprotein complex) to Chl(ide) a is a rather complex process. During conversion of Pchlide a to Chl(ide) a, the chromophore-apoprotein complex undergoes spectral changes the molecular basis of which is not fully understood.
1. Spectral Shift I

Spectral shift I is light-dependent. It was reported by Thorne (1971) in etiolated bean leaves. It occurs as a result of fractional photoconversion of LW t-Pchlide a H (E650 F655), to a dark-stable pigment-apoprotein complex (E668 F674), with a red excitation maximum at 668 nm and a red emission maximum at 655 nm at 77 K. This intermediate yields a mixture of Pchlide and Chlide a after dark-ethanol extraction. The photoconversion rate for Chlide a H (E668 F674) was twice the rate for the photoconversion of the next intermediate, thus suggesting that photoconversion of Pchlide a to Chlide a is a two step two photon process in vivo.
2. Spectral Shift II

Light-dependent spectral shift II was first described by Sironval et al (1967) as a photoconversion of LW t-Pchlide a (E650 F655) to a Chlide a (E676 F690)-apoprotein complex [later on, referred to as (E676 F688) by Sironval and Kuyper (1972)]. Then in 1971, Thorne reported that the photoprecursor of Chlide a (E676 F690) was Chlide a (E668 F674) instead of LW t-Pchlide a (E650 F655). The chemical nature of the chromophore is not clear however. Sironval et al (1967), and Sironval and Kuyper (1972) initially proposed that it was some kind of a Pchlide a-Chlide a intermediate of ill defined nature. Thorne (1971) however, proposed that the chromophore consisted of Chlide a. Shift II was also confirmed by Gassman et al (1968), and Bonner (1969).
3. Spectral Shift III

Shift III takes place very rapidly in darkness. It was considered by Sironval et al (1967) to correspond to the formation of a mature Chlide a-apoprotein complex which releases Chlide a from the Pchlide a oxidoreductase complex. the latter can then pick up another Pchlide a chromophore to yield Pchlide a H (E650 F655).
4. Spectral Shift IV

The shift of Chlide a (E682 F697) to an Chl(ide) a (E672 F680) species was the first spectral shift to be described during the conversion of Pchlide a to Chl(ide) a. It was reported by Shibata in 1957, as a spectral shift that took place in darkness or in the light in about 10 to 20 minutes after the onset of illumination, depending on the age of the etiolated tissue, and the plant species. During this shift Chlide is esterified with geranylgeraniol, which is reduced stepwise to phytol (see reactions between Chlide a and Chl a).
5. Spectral Shift V

The fifth shift was also described by Shibata (1957). It takes place either in the light or in darkness, and corresponds to the final integration of Chl a into various pigment proteins of the thylakoid membranes. On the basis of energy transfer from Pchlide a (E650 F655) to Chlide a (E682 F697) but not to Chl(ide) a (E672 F674), at fractional or partial photoconversions, Thorne (1971) concluded that diffusion of the chromophore from the apoprotein occurs at the level of Chl(ide) a (E674 F683) (shift V) instead of Chlide a (E682 F697) as proposed by Sironval et al (1967). From the maximal appearance of the (E668 F6740) photointermediate and the Pchlide a and Chlide a content Thorne (1971) proposed a size of 20 molecules for the Pchlide a aggregate.
D. Photoreduction of Other Protochlorophyll(ide) a Holochromes.
Under natural photoperiodic greening conditions, Pchlide a and its ester (Pchlide a ester) accumulate during the dark cycles of the photoperiod and contribute to Chl a biosynthesis and accumulation at the onset of light (i. e. at dawn) (Cohen et al, 1977). Although the level of Pchlide a ester drops after the fourth dark cycle (Cohen et al, 1977), it is always detectable in most green plants (Rebeiz, unpublished). Furthermore, both Pchlide a and Pchlide a ester are always present in green tissues during the light phase of the photoperiod (Cohen et al, 1977). During photoperiodic greening Pchlide a (E650 F655), also known as LW t-Pchlide a H [(E450 F657), where E450 refers to the Soret excitation and F657 to the red emission fluorescence maxima at 77 K, as determined on a corrected, high resolution spectrofluorometer] is a transient species which peaks during the 7 th dark cycle and becomes undetectable by the 11 th dark cycle (Cohen and Rebeiz, 1978). On the other hand SW Pchl(ide) a Hs species are present throughout the photoperiodic greening process (Cohen and Rebeiz, 1978). In other words although LW t-Pchlide a H (E450 F657) contributes to prolamellar body reformation and Chl a formation at the end of the dark cycle of the first few dark cycles, it is SW t-Pchlide a Hs, which are prevalent during the dark and light cycles of photoperiodic greening, and which contribute more than LW t-Pchlide a H (E450 F657) to the bulk of Chl a accumulation during photoperiodic greening (Cohen and Rebeiz, 1978).
Of course, the significance to the Chl a biosynthetic process of the accumulation pattern of SW Pchl(ide) a Hs, during normal photoperiodic greening, rests upon the direct photoconvertibilty of SW Pchlide a Hs to Chlide a without conversion to LW t-Pchl(ide a) E450 F657). This was reported to be the case by Cohen and Rebeiz (1978). However the photoconversion was slower than in the case of LW t-Pchlide a H (E450 F650), and took about 10 seconds Cohen and Rebeiz (1978). Since POR B appears to be the prevailing Pchlide a photooxidoreductase under these conditions (Armstrong et al, 1995; Holtorf et al, 1995), it is suggested that the SW t-Pchlide a Hs consist of at least a Pchl(ide) a POR B complex .
Finally, since the Pchlide a chromophore is formed via the DV Pchlide a biosynthetic route in DDV-LDV plants, while it is formed via the MV Pchlide a biosynthetic route in DDV-LMV and DMV-LMV Plants (see Botanical Fall-Outs), it is suggested that SW t-Pchl(ide) a Hs consist of at least a MV Pchlide a chromophore and POR B complex..
E. Kinetics of the photoconversion of the Pchlide a Chromophore to Chlide a
1. Action Spectrum of the Phototransformation
Pchl(ide) a H (E650 F657) is the photoreceptor for its own photoconversion to Chlide a (Koski and Smith, 1951). In an albino corn mutant lacking carotenoids, the action spectrum exhibited two prominent peaks, one at 650 nm and one at 445 nm that corresponded to the absorption spectrum of LW t-Pchlide a H of the mutant.
2. Effect of Temperature on the Phototransformation
The phototransformation of LW t-Pchlide a H to Chlide a was completely inhibited at -195 C (Smith and Benitez, 1954). Partial photoconversion took place at -70 C. At temperatures beyond 50 C, photoconversion was progressively inhibited due to apoprotein denaturation. Dependency of the photoconversion upon temperature indicates that the phototransformation it is not a purely photochemical reaction but also involves a thermochemical component.
3. Quantum Yield of the Phototransformation
The average quantum yield of the photoconversion at 642 and 644 nm amounted to about 0.6 (Smith and Benitez, 1954). Therefore it was not clear from this work whether one or two quanta of light are required for the photoconversion. On the other hands, Thorne (1971) proposed a two quantum process.
4. Effect of Environment on the Phototransformation
The rate of phototransformation expressed as a percentage of the photoconvertible protochlorophyllide was found to be independent of the initial concentration of the holochrome and was not influenced bu the viscosity of the medium (Boardman, 1962). This led to the proposal that the phototransformation did not involve a collision process between independent protein molecules or between a protein molecule and a hydrogen donor molecule. Instead, Boardman (1962) proposed a restricted collision process between the photo activated Pchl molecule and the hydrogen donor. However since the rate of phototransformation was temperature-dependent, it seemed likely that the hydrogenation involved some vibrational of rotational movement of that part of the protein molecule in close proximity to the Pchl.
5. 5. Phototransformation Kinetics
While Smith and Benitez (1954), opted for a bimolecular reaction with respect to Pchl, Boardman and Thorne (1972) suggested that by allowing for energy transfer within molecular groups, the true kinetics of the photoconversion was first order, which is still compatible with the restricted collision hypothesis (Boardman, 1962).
F. Photoconversion of the DV Pchlide a Chromophore to DV Chlide a
A precursor-product relationship between DV Pchlide a and DV Chlide a was established by demonstrating the photoconversion of DV Pchlide a to Chlide a in etiolated cucumber cotyledons induced to accumulate DV Pchlide a exclusively (Duggan and Rebeiz, 1982). Photoconversion of the DV Pchlide a chromophore to DV Chlide a takes place in all plants that accumulate DV Pchlide a (Ioannides et al, 1994). The involvement of PORA and/or PORB in this process is not presently clear.
G. Photoconversion of the MV Pchlide a Chromophore to MV Chlide a
A precursor-product relationship between MV Pchlide a and MV Chlide a was established by demonstrating the photoconversion of MV Pchlide a to MV Chlide a in etiolated cucumber cotyledons enriched in MV Pchlide a (Belanger and Rebeiz, 1980). Photoconversion of the MV Pchlide a chromophore to MV Chlide a takes place in all plants that accumulate MV Pchlide a (Ioannides et al, 1994). The involvement of PORA and/or PORB in this process is not presently clear.
References
- Rebeiz, C. A., S. M. Wu, M. Kuhadja, H. Daniell, and E. J. Perkins. Chlorophyll biosynthetic routes and chlorophyll a chemical heterogeneity in plants. Mol. Cell. Biochem. 58:97-125.
- Rebeiz, C. A., Parham R., Fasoula D.A. and Ioannides I. M. (1994). Chlorophyll a Biosynthetic Heterogeneity. In: Biosynthesis of Tetrapyrroles. Pigments Ciba Symposium 180. pp 177-193, John Wiley, New York.
- Sironval, C., Kuyper, Y., Michel, J. M., and Brouers, M. (1967). The primary photoact in the conversion of protochlorphyll ide into chlorophyllide. Stud. Biophys. 5: 43-50.
- Gassman, M., Granick, S., and Mauzerall, D. (1968). A rapid spectral shift change in etiolated red kidney leaves following phototransformation of protochlorophyllide. Biochem. Biophys. Res. Comm. 32: 295-300.
- Bonner, B. (1969). A short-lived intermediate form in the in vivo conversion of protochlorphyll ide 650 to chlorophyllide 684. Plant Physiol. 44: 739-747.
- Gassman, M. (1973). The conversion of photoinactive protochlorophyllide 633 to phototransformable protochlorophyll ide 650 in etiolated bean leaves treated with delta-aminolevulinic acid. Plant Physiol. 52: 590-594.
- Adamson, H., and Packer, N. (1984). Dark-synthesis of chlorophyll in vivo and dark reduction of protochlorophyll ide in vitro by pea chloroplasts. In: Protochlorophyllide Reduction and Greening. Sironval, C. and Brouers, M. (eds.). pp. 353-363. Martinus Nijhoff, Boston.
- Griffiths, W. T. (1974). Source of reducing equivalents for the in vitro synthesis of chlorophyll from protochlorophyll. FEBS Lett. 46: 301-304.
- Griffiths, W. T. (1978). Reconstitution of chlorophyllide formation by isolated etioplast membranes. Biochem. J. 174: 681-692.
- Apel, K., Santel, H. J., Redlinger, T. E. and Falk, H. (1980). The protochlorophyll ide holochrome of barley (Hordeum vulgare L.). isolation and characterization of the NADPH:protochlorophyll ide oxidoreductase. Eur. J. Biochem. 111: 251-258.
- Apel, K. (1981). The protochlorophyllide holochrome of barley (Hordeum vulgare L.). Phytochrome-induced decrease of the translatable mRNA coding for the NADPH:protochlorophyllide:oxidoreductase. Eur. J. Biochem. 120: 89-93.
- Shulz R., and Senger, H. (1993). protochlorophyll ide Reductase: A keto enzyme in the greening process. In: Pigment-Protein Complexes in Plastids: Synthesis and assembly. Sundqvist, C., and Ryberg, M. (eds.). pp: 179-218. Academic Press, New York.
- Ikeuchi, M., and Murakami, S. (1982). Measurement and identification of NADPH:protochlorophyll ide oxidoreductase solubilized with Triton-X-100 from etioplast membranes of squash cotyledons. Plant Cell. Physiol. 23: 1089-1099.
- Dehesh, K., Hauser, M., and Apel, K. (1986). Light-induced changes in the distribution of the 36,000 Mr polypeptide of NADPH:protochlorophyllide oxidoreductase within different cellular compartments of barley (Hordeum vulgare, L.). I. Localization by immunoblotting in isolated plastids and total extracts. Planta 169: 162-171.
- Santel, H. J., and Apel, K. (1981). The protochlorophyll ide Holochrome of Barley (Hordeum vulgare L.). The effect of light on the NADPH:protochlorophyll ide oxidoreductase. Eur. J. Biochem. 120: 95-103
- Kay, S. A., and Griffiths, W. T. (1983). Light-induced breakdown of NADPH-protochlorophyllide oxidoreductase in vitro. Plant Physiol. 72: 229-236.
- Armstrong, G. A., Runge, S., Frick, G., Sperlink, U., and Apel K. (1995). Identification of NADPH:protochlorophyll ide oxidoreductases A and B: A branched pathway for light-dependent chlorophyll biosynthesis in Arabidopsis thaliana. Plant Physiol. 108: 1505-1517.
- Holtorf, R., Reinbothe, S., Reinbothe, C., Bereza, B. and Apel, K. (1995). Two routes of chlorophyllide synthesis that are differentially regulated by light in barley (Hordeum vulgare L.). Proc. Natl. Acad. Sci. USA 92: 3254-3258.
- Reinbothe C, Apel, K. and Reinbothe S. (1995a). A light-induced protease from barley plastids degrades NADPH:protochlorophyllide oxidoreductase complexed with chlorophyllide. Mol. Cell. Biol. 15: 6206-6212.
- Reinbothe S., Reinbothe C., Holtorf H., and Apel, K. (1995b). Two NADPH:protochlorophyllide oxidoreductases in barley: evidence for the selective disappearance of POR A during the light-induced greening of etiolated seedlings. Plant Cell 7: 1933-1940.
- Cohen, C. E. and Rebeiz, C. A. (1978). Chloroplast Biogenesis XXII. Contribution of short wavelength and long wavelength protochlorophyll species to the greening of higher plants. Plant Physiol. 61:824-829.
- Rebeiz, C. C., and Rebeiz, C. A. (1986). Chloroplast Biogenesis 53: Ultrastructural study of chloroplast development during photoperiodic greening. In: Regulation of Chloroplast Differentiation. Akoyunoglou, G., and Senger, H. (eds.) pp: 389-396. Alan Liss, New York.
- Rebeiz, C. A. (1987). Ever Green. The Sciences.
- Kirk, J. T. O., and Tilney-Basset, R. A. E. (1967). The Plastids: Their Chemistry, Structure, Growth, and Inheritance. pp. 504-506. W. H. Freeman, London.
- Ryberg, M. R., and Sundqvist, C. (1991). Structural and functional significance of pigment-protein complexes of chlorophyll precursors. In: Chlorophylls. Cheer, H. (ed.). pp: 587-612. CRC press, , Florida.
- Tripathy, B. C. and Rebeiz, C. A. Non-equivalence of glutamic acid and delta-aminolevulinic acid as substrates for protochlorophyllide and chlorophyll biosynthesis in darkness. In: Progress in Photosynthesis Research Vol. IV. Biggins, J. (ed.) pp. 439-443. Martinus Nijhoff, Amsterdam.
- Thorne, S. W. (1971). The greening of etiolated bean leaves I. The initial photoconversion process. Biochim. Biophys. Acta. 226: 113-127.
- Sironval, C. and Kuyper, Y. (1972). The reduction of protochlorophyllide into chlorophyllide. Photosynthetica. 6: 254-275.
- Shibata, K. (1957). Spectroscopic studies on chlorophyll formation in intact leaves. J. Biochem. 44: 147-172.
- Cohen, C. E., Bazzaz, M. B., Fullett, S. A., and Rebeiz, C. A. (1977). Chloroplast Biogenesis XX. Accumulation of porphyrin and phorbin pigments in cucumber cotyledons during photoperiodic greening. Plant Physiol. 60: 743-746.
- Koski, V. M., French, C. S., and Smith, J. H. C. (1951). The action spectrum for the transformation of protochlorophyll to chlorophyll in normal and albino corn seedlings. Arch. Biochem. Biophys. 31: 1-17.
- Smith, J. H. C. and Benitez, A. (1954). The effect of temperature on the conversion of protochlorophyll to chlorophyll a in etiolated barley leaves. Plant Physiol. 29: 135-143.
- Boardman, N. K. (1962). Biochim. Biophys. Acta. 64: 279-288.
- Thorne, S. W. and Boardman, N. K. (1972). Biochim. Biophys. Acta. 267: 104-110.
- Ioannides, I., Fasoula D. A., Robertson, K. R. and Rebeiz, C. A. (1994). An evolutionary study of chlorophyll biosynthetic heterogeneity in green plants. Biochem. Syst. Ecol. 22: 211-220.
- Duggan, J. X. and Rebeiz, C. A. (1982). Chloroplast Biogenesis 37. Induction of chlorophyllide a (E459 F675) accumulation in higher plants. Plant Sci. Lett. 24: 27-37.
- Belanger, Faith C. and Rebeiz C. A. (1980b). Chloroplast Biogenesis 30. Chlorophyll(ide) (E459 F675) and Chlorophyll(ide) (E449 F675) the first detectable products of divinyl and monovinyl protochlorophyll photoreduction. Plant Sci. Lett. 18:343-350
Go Back to Main Menu







