 Fatal cases of malaria are mostly caused by infections with Plasmodium falciparum, amounting to ~ 2 million per year worldwide. Increasing resistance to conventional drugs urges for finding new and specific targets in this parasite.
Fatal cases of malaria are mostly caused by infections with Plasmodium falciparum, amounting to ~ 2 million per year worldwide. Increasing resistance to conventional drugs urges for finding new and specific targets in this parasite.
 Fatal cases of malaria are mostly caused by infections with Plasmodium falciparum, amounting to ~ 2 million per year worldwide. Increasing resistance to conventional drugs urges for finding new and specific targets in this parasite.
Fatal cases of malaria are mostly caused by infections with Plasmodium falciparum, amounting to ~ 2 million per year worldwide. Increasing resistance to conventional drugs urges for finding new and specific targets in this parasite.
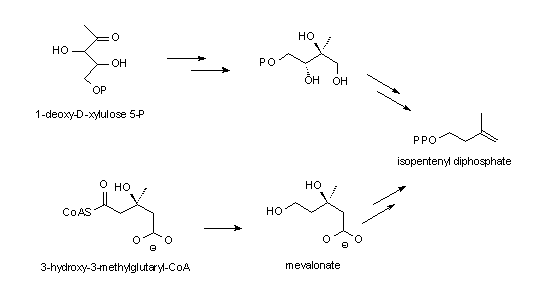
P. falciparum seems to have an uncommon way to synthesize isoprenoids. The key substance, isopentenyl pyrophosphate, is normally synthesized starting from acetyl CoA via 3-hydroxy-3-methylglutaryl coenzyme A (lower path in the panel to the right - termed the mevalonate pathway). Recently an alternative pathway was detected in bacteria, algae, and plants. The naming substance is 1-deoxy-D-xylulose 5-phosphate (DOXP) which arises by condensation of glyceraldehyde 3-phosphate and pyruvate catalyzed by DOXP-synthetase. The next step in this pathway is the formation of 2-C-methyl-D-erythritol 4-phosphate by DOXP reductoisomerase.
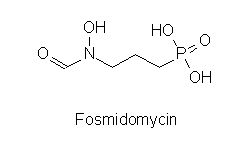
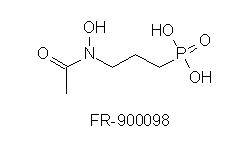
In P. falciparum there is no activity of the mevalonate pathway detectable. Therefore a search for the alternative pathway was initiated, looking for sequences homologous to prokaryotic enzymes. Indeed DOXP reductoisomerase could both be cloned and the dependency of the growth of the parasite on this pathway be demonstrated. The drug fosmidomycin inhibits the P. falciparum DOXP reductoisomerase expressed in E. coli like the enzyme found in bacteria and plants. In a mouse malaria model fosmidomycin administered orally or intraperitoneally after infection prevented parasitemia which untreated is fatal. The methyl substituted drug FR-900098 is effective at even lower concentrations. These results make this class of drugs promising candidates for treatment of human malaria.
 |  |